Scientists Revealed the DNA Cleavage Mechanism of CRISPR-Cas5-HNH Complex
Date:13-02-2025 Print
A groundbreaking study published on Feb 4th reveals new details about the CRISPR-Cas5-HNH/Cascade complex, a variant of the type I-E CRISPR-Cas system, providing critical insights into its DNA recognition and cleavage mechanisms. This complex, which serves as an immune defense system in prokaryotes, has long been studied for its ability to protect bacteria from invading genetic material, such as phages and plasmids. However, recent research uncovers a novel mechanism in the Cas5-HNH/Cascade complex that differs significantly from the well-known type II CRISPR systems like Cas9.
The research team utilized cryo-electron microscopy (cryo-EM) to capture the structures of the Cas5-HNH/Cascade complex in both DNA-bound and unbound states, revealing striking conformational changes. The team’s findings show that the complex adopts a more compact structure when bound to double-stranded DNA (dsDNA), with the target DNA strand making a pronounced U-turn and interacting with the HNH nuclease domain, which cleaves the DNA in a specific order. Interestingly, the target strand is cleaved first, followed by the non-target strand, a departure from the cleavage order seen in other CRISPR systems like Cas12a.
The Cas5-HNH domain itself was found to have a crucial role in the nuclease activity of the complex. The study demonstrated that mutations in key residues of the HNH domain, particularly histidine and aspartate, can completely abolish cleavage activity. This suggests that the HNH domain is not only essential for target DNA recognition but also for the specific cleavage of DNA, further differentiating the mechanism from other systems.
The team also observed that the architecture of the Cas5-HNH/Cascade complex is notably different from canonical type I-E systems. For instance, the HNH domain is fused to the C-terminus of Cas5, replacing the need for Cas3, a protein commonly involved in DNA degradation in other systems. This structural innovation points to the complex’s ability to cleave DNA independently of the traditional components seen in similar CRISPR systems.
The findings of this study offer new insights into the structural dynamics and functional mechanisms of CRISPR-Cas systems, advancing our understanding of bacterial immunity and providing a foundation for potential innovations in CRISPR-based genome-editing technologies.
By elucidating the role of the HNH nuclease domain and revealing the structural changes that occur upon DNA binding, this work not only deepens our understanding of CRISPR-Cas5-HNH but also paves the way for refining CRISPR technologies for therapeutic applications.
This study entitled “Structural basis for RNA-guided DNA degradation by Cas5-HNH/Cascade complex” was published on Nature Communications.
The study was supported by the National Science Foundation and the Chinese Academy of Sciences.
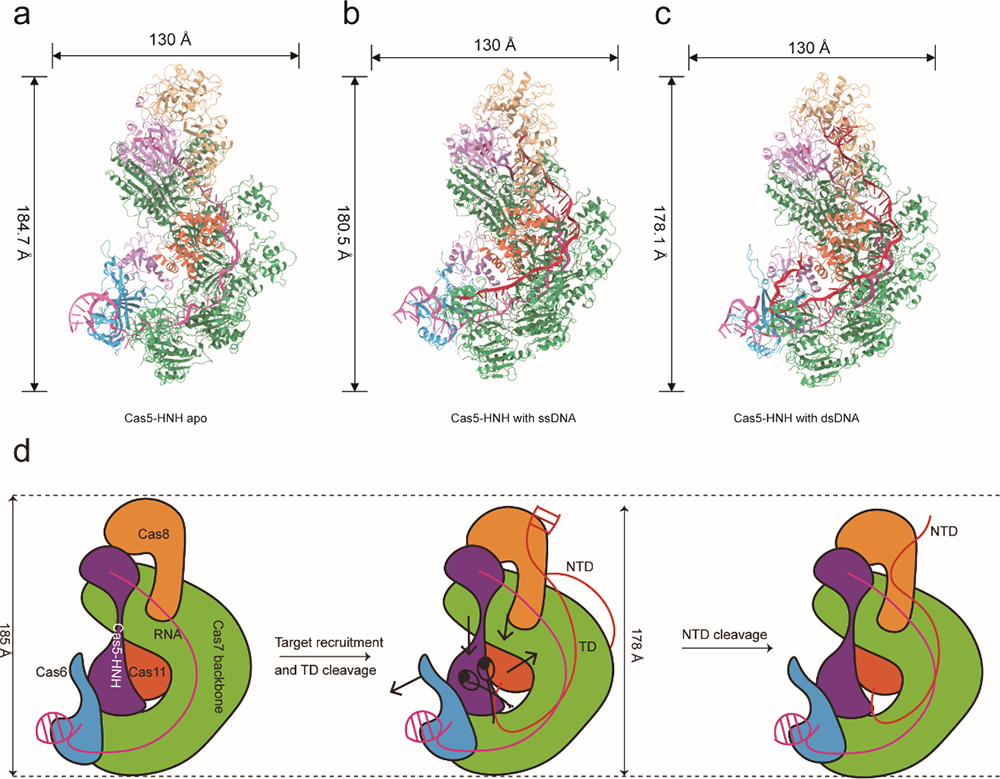
Fig.1 Structures of the CRISPER-Cas5-HNH complex and the proposed mechanism (Image by Institute of Physics).
Contact:
Institute of Physics
ZHU Hongtao
Email:hongtao.zhu@iphy.ac.cn
Key words:
Cryo-EM, Phage immunity, DNA cleavage, CRISPER
Abstract:
Hongtao Zhu group from Institute of Physics uncovers new details about the CRISPR-Cas5-HNH/Cascade complex, a variant of the type I-E CRISPR system, offering insights into its DNA recognition and cleavage mechanisms. Using cryo-electron microscopy, the researchers captured the structures of the complex in both DNA-bound and unbound states, revealing significant conformational changes. Upon binding DNA, the complex adopts a compact structure, with the target strand interacting with the HNH nuclease domain, which cleaves the target strand first, followed by the non-target strand—an order distinct from other CRISPR systems like Cas12a. Mutagenesis of key residues in the HNH domain confirmed its essential role in both DNA recognition and cleavage. Additionally, the study revealed that the HNH domain’s fusion to the C-terminus of Cas5 eliminates the need for Cas3, a key component in other systems. These findings enhance our understanding of CRISPR-Cas5-HNH and inform potential therapeutic applications.


