Electrically driven phase transformation in cerium oxides and the related properties studied by in situ TEM
Date:07-04-2010 Print
Cerium oxides are cubic fluorite structure, in which cerium cation sublattice is a superstable frame while oxygen anions are active and have higher mobility. Cerium oxides have been attracting much attention because of their uses in three-way catalyst and other catalyst applications. Redox reaction of cerium oxides, as the basis of their uses as catalyst, usually takes place at high temperature (> 600 K) and/or low oxygen partial pressure. There have been continuous efforts to further lower the operating temperatures of cerium oxide for improving performance of the catalysts and reducing pollution during the cold-start condition.
In past several years, Professor Xuedong Bai’s group in institute of Physics, Chinese Academy of Sciences (CAS), has been working on the properties of low-dimensional materials with well-defined structure, by building a nanomanipulator inside transmission electron microscope (TEM). Based on the in situ TEM technique, they have made progress on probing novel properties of individual nanostructures. The related results have been published in the international journals, such as JACS 131, 62 (2009), JMC 19, 1002 (2009), and APL 92, 213105 (2008) etc. Recently, Professor Enge Wang and his students Peng Gao and Wangyang Fu, Dr. Zhenchuan Kang, Associate professor Wenlong Wang, and Professor Xuedong Bai have achieved a direct atomic-scale observation of a redox process in cerium oxides driven by electric field at ambient temperature. The dynamic changes taking place during the electrically driven redox reaction are imaged by in situ high-resolution TEM, where reversible phase transformations due to migration of oxygen vacancies have been reproducibly achieved. It is found that, in addition to temperature and oxygen partial pressure, electric field is verified as a new parameter that can efficiently induce the phase transformation of cerium oxides. These results could lead to low-temperature operation of catalysts for purification of automobile emissions of pollutants, oxygen generation, and intermediate-temperature solid oxide fuel cells. This work is published as an article on JACS 132, 4197-4201 (2010).
Furthermore, they used in situ TEM method to study the role of oxygen vacancies for the resistance switching effect in cerium oxides. The structure change during oxygen vacancy migration in CeO2 induced by electric field was in situ imaged inside TEM, which gives a direct evidence for oxygen migration mechanism for the microscopic origin of resistance change effect in CeO2. Our results have implications for understanding the nature of resistance change in metal oxides with mixed valence cations. This work is published on Micron 41, 301-305 (2010).
The authors acknowledge financial supports from the NSF, MOST, and CAS of China.
References:
1."Electrically driven redox process in cerium oxides",JACS 132, 4197-4201 (2010).
2."In situ TEM studies of oxygen cacancy migration for electrically induced resistance change effect in cerium oxides", Micron 41, 301-305 (2010).
In past several years, Professor Xuedong Bai’s group in institute of Physics, Chinese Academy of Sciences (CAS), has been working on the properties of low-dimensional materials with well-defined structure, by building a nanomanipulator inside transmission electron microscope (TEM). Based on the in situ TEM technique, they have made progress on probing novel properties of individual nanostructures. The related results have been published in the international journals, such as JACS 131, 62 (2009), JMC 19, 1002 (2009), and APL 92, 213105 (2008) etc. Recently, Professor Enge Wang and his students Peng Gao and Wangyang Fu, Dr. Zhenchuan Kang, Associate professor Wenlong Wang, and Professor Xuedong Bai have achieved a direct atomic-scale observation of a redox process in cerium oxides driven by electric field at ambient temperature. The dynamic changes taking place during the electrically driven redox reaction are imaged by in situ high-resolution TEM, where reversible phase transformations due to migration of oxygen vacancies have been reproducibly achieved. It is found that, in addition to temperature and oxygen partial pressure, electric field is verified as a new parameter that can efficiently induce the phase transformation of cerium oxides. These results could lead to low-temperature operation of catalysts for purification of automobile emissions of pollutants, oxygen generation, and intermediate-temperature solid oxide fuel cells. This work is published as an article on JACS 132, 4197-4201 (2010).
Furthermore, they used in situ TEM method to study the role of oxygen vacancies for the resistance switching effect in cerium oxides. The structure change during oxygen vacancy migration in CeO2 induced by electric field was in situ imaged inside TEM, which gives a direct evidence for oxygen migration mechanism for the microscopic origin of resistance change effect in CeO2. Our results have implications for understanding the nature of resistance change in metal oxides with mixed valence cations. This work is published on Micron 41, 301-305 (2010).
The authors acknowledge financial supports from the NSF, MOST, and CAS of China.
References:
1."Electrically driven redox process in cerium oxides",JACS 132, 4197-4201 (2010).
2."In situ TEM studies of oxygen cacancy migration for electrically induced resistance change effect in cerium oxides", Micron 41, 301-305 (2010).
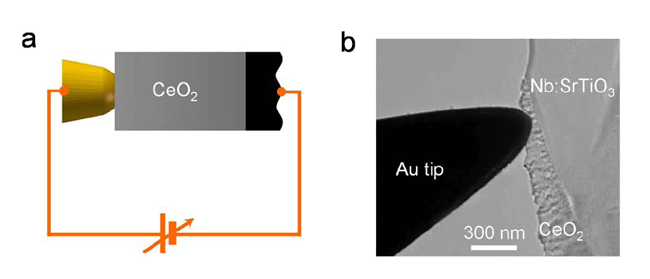 |
| Figure 1. (a) Scheme of the experimental setup. (b) In situ TEM image of the gold tip-CeO2 film-conductive substrate structure. |
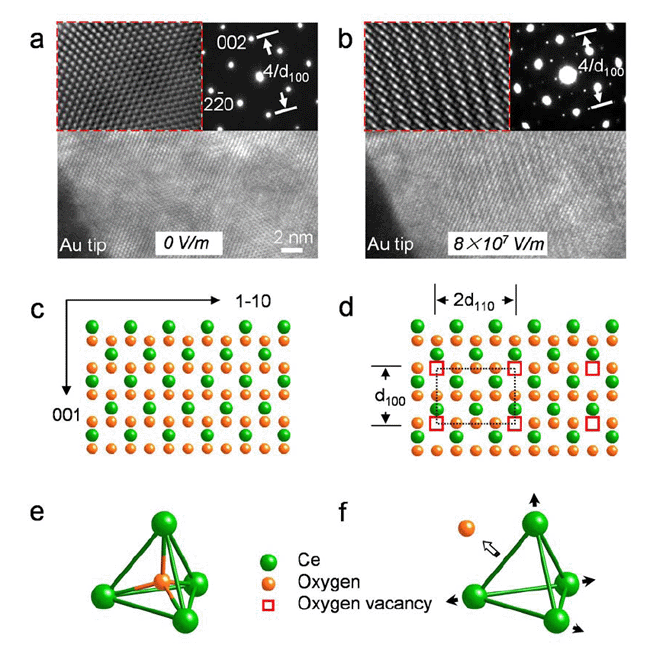 |
| Figure 2. Oxygen vacancy migration in cerium oxides driven by electric field. |
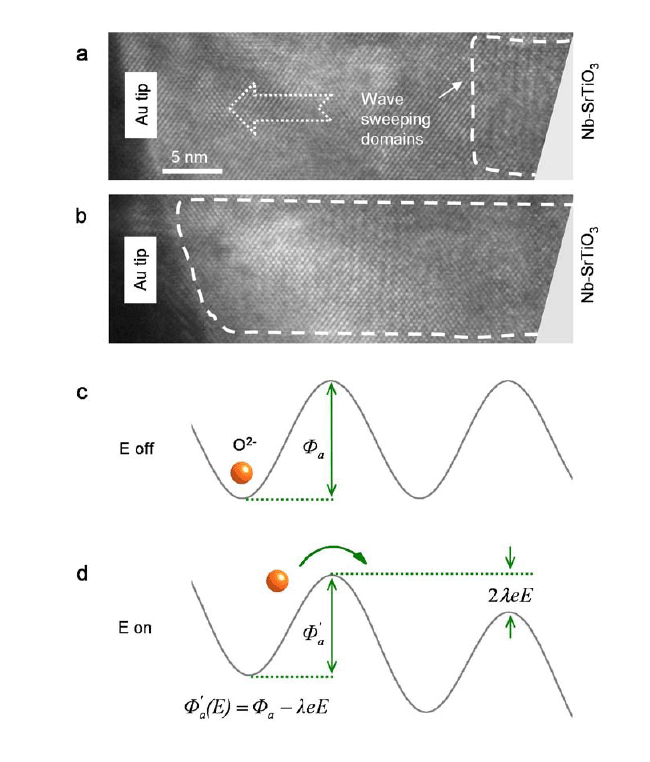 |
| Figure 3. Kinetic process of oxygen vacancy migration in cerium oxides driven by electric field. |
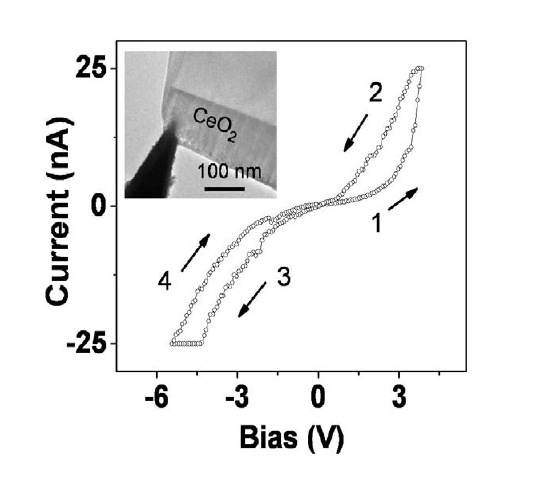 |
| Figure 4. Resistance switching effect in cerium oxides. |
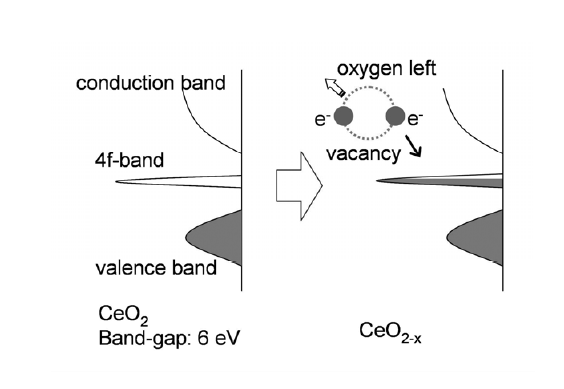 |
| Figure 5. Mechanism on electrically induced resistance change effect in cerium oxides. |


