Progress in exploration of new functional borate crystals
Date:25-08-2010 Print
Borates have received continuing interests due to the rich varieties of structures as well as their excellent physical/chemical properties such as wide optical transmittance, high damage threshold, good thermal and chemical stability, etc. Over the past 50 years, more than one thousand new functional borates have been found as potential nonlinear optical crystals, luminescent materials, laser crystals and so on, which makes borates one of the most important classes of compounds for exploration of new functional materials.
Prof. Xiaolong Chen and his colleagues from the Research & Development Center for Functional Crystals, Institute of Physics, Chinese Academy of Sciences focused on new borates since 1990s in search of new materials. Many new functional borate crystals were found through phase relation study combing with powder or single crystal X-ray diffraction structural analyses. Among them, YBa3B9O18 is a promising material for thin disk laser with unobvious quenching effect though the concentrations of rare earth ion are very high. (K1-xNax)2Al2B2O7 (0 ≤ x < 0.6) and MM'4(BO3)3 (M = Na, M' = Ca, M = K, M' = Ca, Sr) series are hopeful nonlinear optic materials, and high quality crystals can be obtained. LiSr4B3O9 crystallizes in the cubic space group with high symmetry that is rarely unusual for borates. The results presented above were published in 【Inorganic Chemistry 43, 8555 (2004), 44, 6409 (2005), 45, 3042 (2006) and Chemistry of Materials 7, 2193 (2005)】.
On the basis of the borate structures discovered, researchers postulated several rules as the foundation of borate structural chemistry: 1. boron binds to either three or four oxygen atoms to form a BO3 triangle or a BO4 tetrahedron; 2. BO3 and BO4 groups can only link to each other via common corners, not by edge-sharing or face-sharing; 3. corner-sharing B-O blocks form the fundamental building blocks (FBB, the repeat B-O block of the structure). The formation of corner-sharing B-O blocks is a direct derivative of the Pauling's 3rd and 4th rules. Recently, Huppertz et al. gave the first violation of this hypothesis by synthesizing Dy4B6O15 under high pressure (HP) (8 GPa, 1000 K) 【J. Am. Chem. Soc 124,9376 (2002) 】. However, the material is unstable at ambient conditions. Therefore, edge-sharing B-O blocks are only considered to be the extension of borate structural chemistry under extreme conditions. Very recently, Dr. Shifeng Jin, Prof. Xiaolong Chen et al. synthesized a novel borate KZnB3O6 under ambient pressure, which is built from edge-sharing BO4 tetrahedra and is stable up to its melting point. This work demonstrates that high pressure is not an indispensable prerequisite for the formation of edge-sharing BO4 polyhedra, and that the original hypothesis should be re-examined.
Figure 1 shows the structure of KZnB3O6, in which the blue polyhedral consists of two edge-sharing BO4 tetrahedra and four corner-sharing BO3 triangles (Fig. 1c). Although the synthesis condition and coordination environment of B-O groups are very different for previously reported HP-borates and the title compound, the geometry of those edge-sharing tetrahedral is highly consistent. As a general rule, the presence of shared edges or faces of polyhedra centred by high-valence low coordinated small cations (B, P, Si etc.) will increase the formation energy and result in instability in structure. The calculation of deformation charge density also revealed that the bond strength of edge-sharing region is a lot weaker than that of the corner-sharing region. However, KZnB3O6 can survive up to its melting point, indicating that it’s the first stable edge-sharing borate in ambient pressure. Furthermore, the crystal structure is well preserved from room temperature down to 30K (right of Fig. 1). Through the geometry optimization and total energy calculation, we found that it is energetically more favorable for KZnB3O6 to retain its novelty rather than adopting the common corner-sharing one (Fig 2). Interestingly, the indispensable K cations within the structure are mobile and partially exchangeable with the original crystal morphology preserved, which demonstrates the edge-sharing framework is very stable. The result has been published in【Angew. Chem. Int. Ed. 49, 4967 (2010)】.
This work was financially supported by the National Natural Science Foundation of China, the International Centre for Diffraction Data (ICDD, USA) and so on.
Prof. Xiaolong Chen and his colleagues from the Research & Development Center for Functional Crystals, Institute of Physics, Chinese Academy of Sciences focused on new borates since 1990s in search of new materials. Many new functional borate crystals were found through phase relation study combing with powder or single crystal X-ray diffraction structural analyses. Among them, YBa3B9O18 is a promising material for thin disk laser with unobvious quenching effect though the concentrations of rare earth ion are very high. (K1-xNax)2Al2B2O7 (0 ≤ x < 0.6) and MM'4(BO3)3 (M = Na, M' = Ca, M = K, M' = Ca, Sr) series are hopeful nonlinear optic materials, and high quality crystals can be obtained. LiSr4B3O9 crystallizes in the cubic space group with high symmetry that is rarely unusual for borates. The results presented above were published in 【Inorganic Chemistry 43, 8555 (2004), 44, 6409 (2005), 45, 3042 (2006) and Chemistry of Materials 7, 2193 (2005)】.
On the basis of the borate structures discovered, researchers postulated several rules as the foundation of borate structural chemistry: 1. boron binds to either three or four oxygen atoms to form a BO3 triangle or a BO4 tetrahedron; 2. BO3 and BO4 groups can only link to each other via common corners, not by edge-sharing or face-sharing; 3. corner-sharing B-O blocks form the fundamental building blocks (FBB, the repeat B-O block of the structure). The formation of corner-sharing B-O blocks is a direct derivative of the Pauling's 3rd and 4th rules. Recently, Huppertz et al. gave the first violation of this hypothesis by synthesizing Dy4B6O15 under high pressure (HP) (8 GPa, 1000 K) 【J. Am. Chem. Soc 124,9376 (2002) 】. However, the material is unstable at ambient conditions. Therefore, edge-sharing B-O blocks are only considered to be the extension of borate structural chemistry under extreme conditions. Very recently, Dr. Shifeng Jin, Prof. Xiaolong Chen et al. synthesized a novel borate KZnB3O6 under ambient pressure, which is built from edge-sharing BO4 tetrahedra and is stable up to its melting point. This work demonstrates that high pressure is not an indispensable prerequisite for the formation of edge-sharing BO4 polyhedra, and that the original hypothesis should be re-examined.
Figure 1 shows the structure of KZnB3O6, in which the blue polyhedral consists of two edge-sharing BO4 tetrahedra and four corner-sharing BO3 triangles (Fig. 1c). Although the synthesis condition and coordination environment of B-O groups are very different for previously reported HP-borates and the title compound, the geometry of those edge-sharing tetrahedral is highly consistent. As a general rule, the presence of shared edges or faces of polyhedra centred by high-valence low coordinated small cations (B, P, Si etc.) will increase the formation energy and result in instability in structure. The calculation of deformation charge density also revealed that the bond strength of edge-sharing region is a lot weaker than that of the corner-sharing region. However, KZnB3O6 can survive up to its melting point, indicating that it’s the first stable edge-sharing borate in ambient pressure. Furthermore, the crystal structure is well preserved from room temperature down to 30K (right of Fig. 1). Through the geometry optimization and total energy calculation, we found that it is energetically more favorable for KZnB3O6 to retain its novelty rather than adopting the common corner-sharing one (Fig 2). Interestingly, the indispensable K cations within the structure are mobile and partially exchangeable with the original crystal morphology preserved, which demonstrates the edge-sharing framework is very stable. The result has been published in【Angew. Chem. Int. Ed. 49, 4967 (2010)】.
This work was financially supported by the National Natural Science Foundation of China, the International Centre for Diffraction Data (ICDD, USA) and so on.
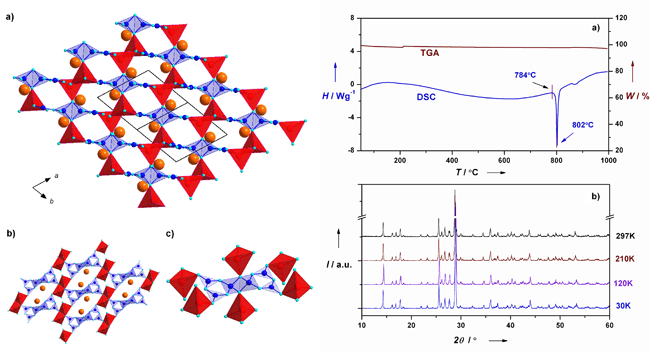 |
| Fig. 1 Crystal structure of KZnB3O6 (left) and its thermal stability (right). |
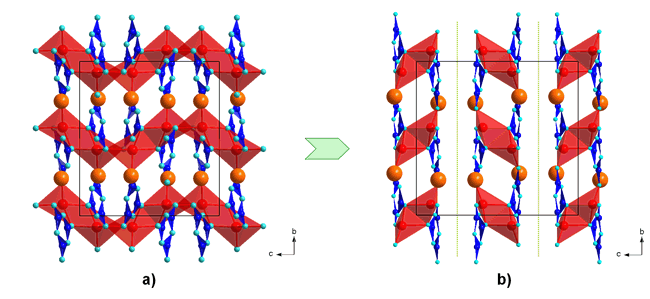 |
| Fig. 2 Structure relaxation results of suppositional common lattice type of KZnB3O6. |


