New non-iodide redox couples for dye-sensitized solar cells
Date:10-01-2011 Print
Dye-sensitized solar cell (DSC) has established itself a representative thin film photovoltaic device since it offers the prospect of simple operation, low-cost fabrication and relatively high conversion efficiency. In recent years, Professor Qingbo Meng’ group from Institute of Physics (IOP), Chinese Academy of Sciences (CAS), has been working on developing key materials of the DSCs and the technical progress to accelerate the practical application.
By close collaboration with Academician Liquan Chen, Professors Hong Li and Zhaoxiang Wang from the IOP, CAS, some fascinating results have been achieved, such as solid-state electrolytes based on mono-iodide ion conductors, low-cost and environmentally friendly electrolytes and polymer-based electrolytes etc.(Chem. Commun., 2004, 2186;J. Am. Chem. Soc., 2005, 127, 6394;Electrochem. Commun., 2006, 8, 170;J. Am. Chem. Soc.,2006, 128, 8720;Energy Environ. Sci., 2009, 2, 283;Langmuir, 2009, 25, 4808;Electrochimica Acta 2010, 55, 895;Energy Environ. Sci., 2011, 3, in press).
Meanwhile, novel counter electrodes based on new carbon materials or conductive polymers have also been developed for further reducing the the cost of DSCs(Electrochem. Commun., 2007, 9, 596;Electrochem. Commun. 2009, 11, 1346;Carbon 2009, 47, 2704;J. Phys. Chem. C, 2010,114,11673). All these work have received widely attention.
So far, I3– /I– redox couple is the most popular charge mediator for DSCs. Unfortunately, they are not the ideal mediator due to the following negative features: (1) I2 is corrosive toward most metals, leading to poor long-term stability, technical complexity of the cell and thus increasing the device cost. Especially when the silver grid is adopted as the current collection to decrease the sheet resistance of a large scale device, the I3– /I– couple will gradually dissolve this metal grid. Extra protection of the silver grid against this corrosion is thus needed. (2) I2 has a substantial vapor pressure, which will cause potential unstability unless perfectly sealed. (3) I3– /I– couple can absorb a significant amount of visible light. (4) The redox potential of I3– /I– couple limits further improvement of maximum open circuit photovoltage (Voc). Therefore, seeking appropriate non-corrosive new redox couples are very important issue for the future of DSCs.
Recently, Dr. Dongmei Li et.al from IoP, CAS worked together with Prof. M. Armand from Université de Picardie Jules, France, to develop a new colorless organic non-iodide redox couple for DSCs (tetramethylthiourea (TMTU) and tetramethylformaminium disulphide dication ([TMFDS]2+). This redox couple exhibits some attractive advantages, such as cheapness, easy handling, non-corrosion and no visible-light absorption. The most important is that it is compatible with cheap carbon counter electrodes and metal sheet substrates. The [TMFDS]2+/TMTU-based DSCs can present 4.5% of efficiency at AM 1.5 illumination, distinctly outperform the identical DSCs with Pt electrode. Higher Voc can be prospectively achieved by systematical optimization of the device. Taking account into the general interest of DSCs, this work opens a new way to develop low-cost, stable non-iodine photovoltaic devices, promotes practical applications of DSCs. Meanwhile, it provides a new method to in-depth understand the charge transfer mechanism in DSCs as well.
Related results were published in Adv. Funct. Mater., 2010, 20, 3358. The work was supported by NSFC, CAS and MOST.
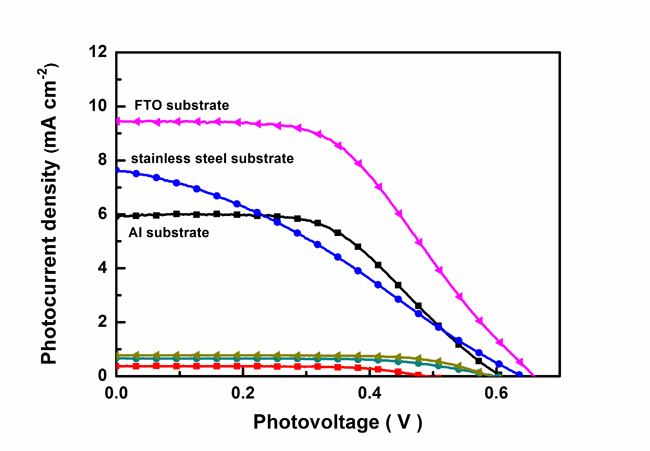 |
| Photocurrent density-photovoltage characteristics for DSCs with carbon counter electrodes on different substrates under AM 1.5 irradiation. |


