High-efficiency and bulk-quantity synthesis of B and N co-doped single-walled carbon nanotubes through improved carbothermal substitution reaction
Date:23-11-2011 Print
Substitutional doping of carbon nanotubes (C-NTs) with boron (B) and/or nitrogen (N) to tune their electronic properties is of fundamental and practical importance. Take the B and N co-doped ternary system BCN-NTs for instance, both theoretical predications and experimental evidences have revealed that they would exhibit the tunable semiconducting properties intermediate between those of metallic or small-gap C-NTs and insulating BN-NTs, as in contrast to the case of pristine C-NTs that naturally consist of two-thirds semiconducting and one-third metallic NTs. However, research to date has been severely hindered by the challenging difficulties involved in synthesis of the ternary BCN-NTs, especially the single-walled NTs (SWNTs).
Recently, Prof. BAI Xuedong’s group from Institute of Physics, Chinese Academy of Sciences, and collaborator in Peking University, succeeded in developing an innovative wet chemistry-assisted carbothermal substitution reaction approach for the high-efficiency and bulk-quantity synthesis of ternary BCN-SWNTs by employing pristine C-SWNTs as the templating precursors. The as-synthesized BCN-SWNTs are of high purity and quality rivaling that of the starting C-SWNTs. Combined TEM and EELS analyses together with XPS, FT-IR, and Raman spectroscopic studies confirmed the true substitutional incorporation of B and N dopants into the graphitic tube-shell lattice, with the single-layered nanotubular topology being well-preserved. As a consequence of B/N codoping, the electronic properties of the pristine C-SWNTs were successfully modified, and the thin-film electrical devices built from BCN-SWNTs exhibited good electrical performance characterized by semiconducting behavior. It is expected that the capability of this wet-chemistry-assisted substitution reaction approach is potentially extendable to the B/N doping of other graphene-related materials.
Over the past a few years, the same group of authors has been engaged in BCN-NT research and published several important papers, e.g. J. Am. Chem. Soc.(2006, 128, 6530)and Adv. Mater. (2008, 20, 3615). This work is as a continuation of their ongoing efforts and the present results was recently published in J. Am. Chem. Soc.(2011,133, 13216).
This work is supported by Chinese Academy of Sciences, the National Natural Science Foundation of China, and the Chinese Ministry of Science and Technology.
Recently, Prof. BAI Xuedong’s group from Institute of Physics, Chinese Academy of Sciences, and collaborator in Peking University, succeeded in developing an innovative wet chemistry-assisted carbothermal substitution reaction approach for the high-efficiency and bulk-quantity synthesis of ternary BCN-SWNTs by employing pristine C-SWNTs as the templating precursors. The as-synthesized BCN-SWNTs are of high purity and quality rivaling that of the starting C-SWNTs. Combined TEM and EELS analyses together with XPS, FT-IR, and Raman spectroscopic studies confirmed the true substitutional incorporation of B and N dopants into the graphitic tube-shell lattice, with the single-layered nanotubular topology being well-preserved. As a consequence of B/N codoping, the electronic properties of the pristine C-SWNTs were successfully modified, and the thin-film electrical devices built from BCN-SWNTs exhibited good electrical performance characterized by semiconducting behavior. It is expected that the capability of this wet-chemistry-assisted substitution reaction approach is potentially extendable to the B/N doping of other graphene-related materials.
Over the past a few years, the same group of authors has been engaged in BCN-NT research and published several important papers, e.g. J. Am. Chem. Soc.(2006, 128, 6530)and Adv. Mater. (2008, 20, 3615). This work is as a continuation of their ongoing efforts and the present results was recently published in J. Am. Chem. Soc.(2011,133, 13216).
This work is supported by Chinese Academy of Sciences, the National Natural Science Foundation of China, and the Chinese Ministry of Science and Technology.
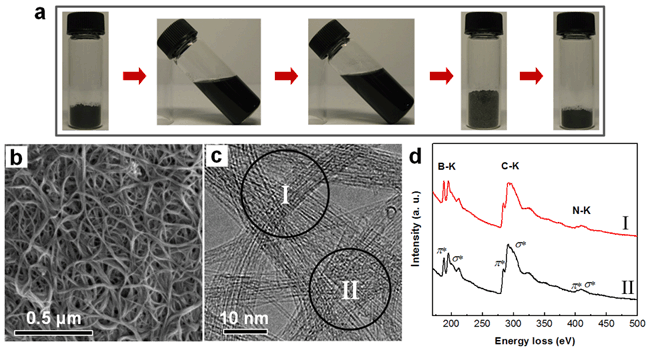 |
| Figure 1. (a) Photographs showing the processing sequence of SWNTs. From left to right: the starting HiPCO C-SWNTs, suspension of the exfoliated SWNTs in BMIBF4, suspension after the mixing of boric acid, boric acid wrapped SWNTs, and the resultant BxCyNz-SWNTs after carbothermal substitution reaction at 1000℃. (b and c) Typical SEM and TEM images of the as-synthesized BxCyNz-SWNTs. (d) Corresponding EELS spectra recorded from the two different probe positions as marked in c. (image by BAI Xuedong et al.) |
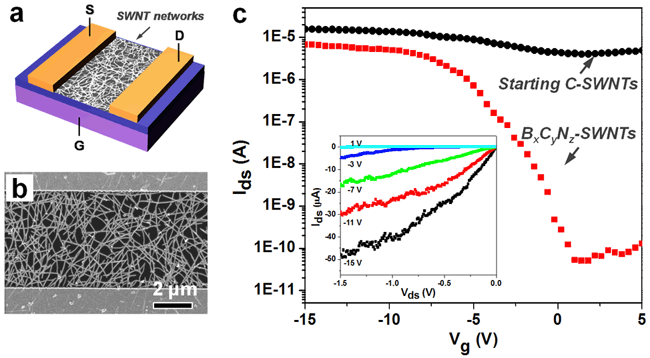 |
| Figure 2. (a) Schematic of device geometry of the SWNT network TFT, with Ti/Au (2 nm/70 nm) source and drain contacts and SiO2(300 nm) gate dielectric. (b) SEM image of a typical device with ~5μm channel length. (c) Representative room-temperature Ids-Vgtransfer characteristics recorded at Vds=0.1 V for two devices built from dense percolating networks of the BxCyNz-SWNTs and the starting C-SWNTs, respectively. The inset is Ids-Vdsoutput characteristics at various Vgfor the BxCyNz-SWNT device. To ensure a fair comparison of electronic analysis, the SWNT densities and device geometries are controlled to be nearly identical for these devices. (image by BAI Xuedong et al.) |


