Finding toughening mechanisms of metallic glasses
Date:07-12-2011 Print
It has been a long outstanding question on whether atomic size effects or electronic structure plays the major role of plasticity versus brittleness properties of metallic alloys.
Recently, Chenchen Yuan, Junfeng Xiang, Xuekui Xi, and Weihua Wang, in Institute of Physics of Chinese Academy of Sciences employed monolithic metallic glass (MG) as a model alloy system to attack the above question by fine tuning plasticity versus brittleness upon microalloying, while at the same time monitoring their electronic properties by 27Al NMR microscopy. The major finding is that the measured isotropic 27Al NMR shifts track the measured fracture energy. Since the isotropic 27Al NMR shifts for these non-magnetic MG alloys are mainly determined by the s character of the electron density of states at the Fermi level, gs(Ef), that means the larger gs(Ef), the higher fracture energy of these MGs. The researchers further proposed that the bond mobility or directionality of Al with neighboring transition-metal atoms might govern the fracture energy dissipation. Their proposal can be verified by recent quantum mechanical computations on intermetallic compounds and MGs, respectively. The calculations indicate a strong directional bonding character due to the hybridized Al 3p and TM (TM = Zr, Ti, Cu, Ni) d orbitals evidenced by the distribution of the density of states at energy scales.
The NMR result of strong correlation of NMR shifts with fracture energy strongly supports the electronic origin of microalloying induced embrittlement in alloys and compounds and also reveals the nature of bond mobility mechanism in the MGs. This work was published in recent issue of Phys review letters. PRL 107, 236403 (2011).
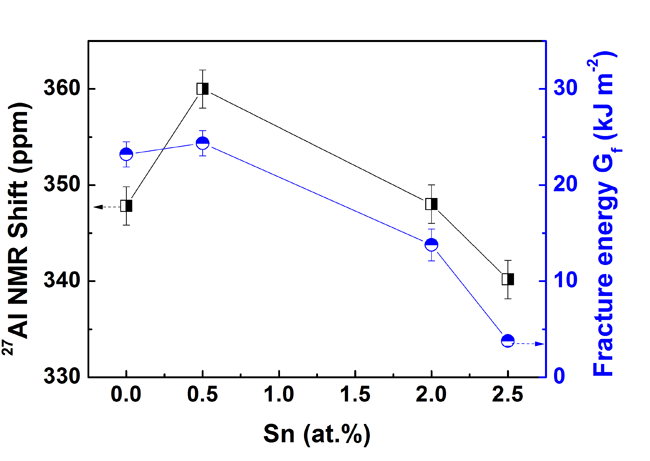 |
| Fig. 1 The correlation of27Al NMR shifts (left coordinate) and fracture energy (right coordinate) with Sn concentration. Image by C.C. Yuan,et al. |
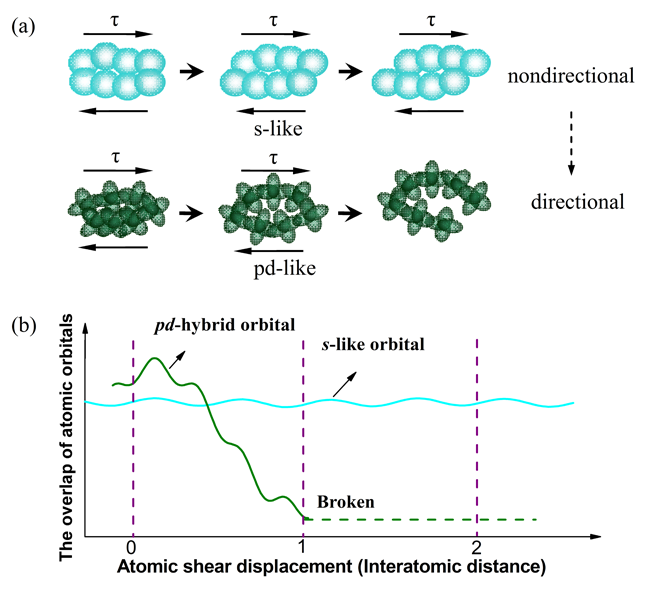 |
| Fig. 2 (a) A schematic illustration of the mobility of non-directional versus directional bonds during shear; (b) the overlap of atomic orbital versus the atomic shear displacement. Image by C.C. Yuan,et al. |
Article linkage: http://link.aps.org/doi/10.1103/PhysRevLett.107.236403


