Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery
Date:09-10-2012 Print
In the field of energy storage, lithium (Li)-ion batteries dominate the portable consumer electronic market because of their high energy density and have also been extensively explored as power sources for various types of important applications, such as electrical vehicles and smart grid. Due to the low abundance of lithium in the earth’s crust (0.0065%), large-scale applications of lithium ion batteries become questionable, especially if low cost and high efficient recycling technology could not be developed. Alternatively, sodium (Na)-ion batteries have again aroused increasing interest for large-scale energy storage, particularly as stationary batteries for smart grid, solar and wind power, owing to a large abundance of sodium, for which the demanding on energy densities of the power sources is not so seriously.
Recently, PhD student ZhAO Liang, Prof. HU Yongsheng et al. in Group E01 from the Institute of Physics, Chinese Academy of Sciences, proposed a low-cost carboxylate-based organic material, disodium terephthalate (Na2C8H4O4), as a novel anode material for low-cost room-temperature Na-ion batteries. To the best of our knowledge, this is the first time that an organic compound is reported for the use as an anode material for Na-ion batteries. This material exhibits a low Na storage voltage at 0.43 V vs. Na+/Na and a high reversible capacity of 250 mAh/g, corresponding to a two-electron transfer, with excellent cycling performance. By performing ALD Al2O3 coating on the surface of the Na2C8H4O4/KB electrode, Na storage performance is significantly improved in terms of initial coulombic efficiency, rate capability, and cycling performance. The results were published on Advanced Energy Materials (Adv. Energy Mater., 2012, 2: 962-965.).
The above work was supported by funding from the National High Technology Research and Development Program of China (Grant No. 2009AA033101), the National Basic Research Program of China (Grant No. 2009CB220104), the National Natural Science Foundation of China (Grant No.50972164), Chinese Academy of Sciences Project (Grant No. KJCX2-YW-W26).
The link for the paper:
1.“Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery”
http://onlinelibrary.wiley.com/doi/10.1002/aenm.201200166/pdf
Recently, PhD student ZhAO Liang, Prof. HU Yongsheng et al. in Group E01 from the Institute of Physics, Chinese Academy of Sciences, proposed a low-cost carboxylate-based organic material, disodium terephthalate (Na2C8H4O4), as a novel anode material for low-cost room-temperature Na-ion batteries. To the best of our knowledge, this is the first time that an organic compound is reported for the use as an anode material for Na-ion batteries. This material exhibits a low Na storage voltage at 0.43 V vs. Na+/Na and a high reversible capacity of 250 mAh/g, corresponding to a two-electron transfer, with excellent cycling performance. By performing ALD Al2O3 coating on the surface of the Na2C8H4O4/KB electrode, Na storage performance is significantly improved in terms of initial coulombic efficiency, rate capability, and cycling performance. The results were published on Advanced Energy Materials (Adv. Energy Mater., 2012, 2: 962-965.).
The above work was supported by funding from the National High Technology Research and Development Program of China (Grant No. 2009AA033101), the National Basic Research Program of China (Grant No. 2009CB220104), the National Natural Science Foundation of China (Grant No.50972164), Chinese Academy of Sciences Project (Grant No. KJCX2-YW-W26).
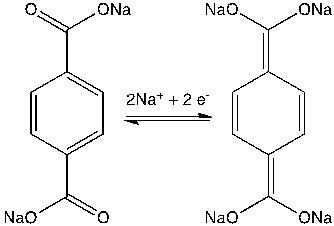 |
| Figure 1. Molecular structure of disodium terephthalate (Na2C8H4O4) and the Na insertion/deinsertion mechanism. (Image by HU Yongsheng et al.) |
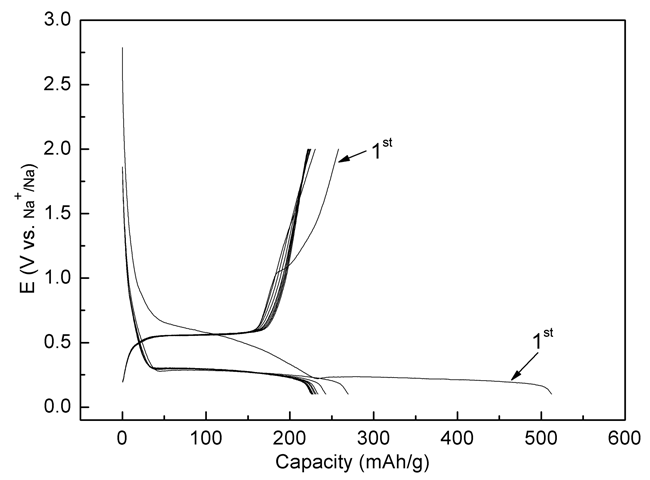 |
| Figure 2. The first 10 discharge-charge cycles of the Na2C8H4O4/KB composite electrode, the voltage range is between 0.1 V and 2 V. (Image by HU Yongsheng et al.) |
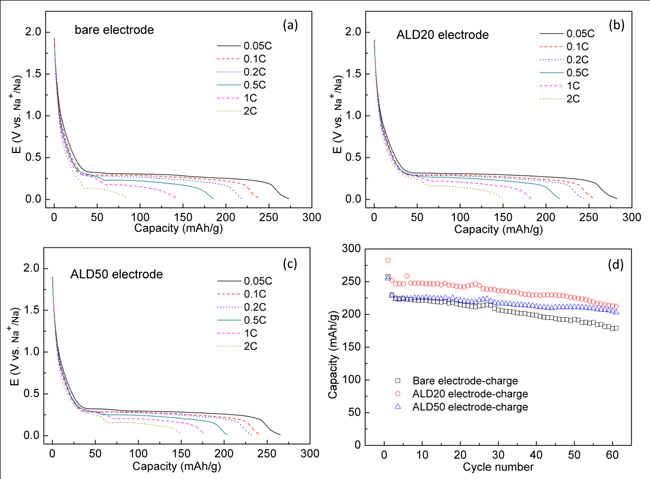 |
| Figure 3. Discharge curves of the untreated Na2C8H4O4electrode (a) and the ALD20 (ca. 2nm Al2O3coating) (b) and ALD50 (ca. 5nm Al2O3coating) (c) electrodes at different discharge-charge current rates, the curves shown here are the end ones of each five cycles. (d) The cycling performance of the untreated Na2C8H4O4electrode and the ALD20 and ALD50 electrodes tested at 0.1C, the voltage range is between 0.01 V and 2 V. (Image by HU Yongsheng et al.) |
The link for the paper:
1.“Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery”
http://onlinelibrary.wiley.com/doi/10.1002/aenm.201200166/pdf


