Kinetic Origin of Divergent Decompression Pathways in Silicon and Germanium
Date:10-05-2013 Print
Group IV elemental (C, Si, Ge) solids exhibit a rich variety of pressure induced structural phase transitions. While they share many structural features because of their common covalent bonding nature, they also show distinct characteristics that reflect their subtle differences in the underlying physics that govern the energetic and kinetic aspects of the phase transformation process. At ambient conditions carbon exists in the form of graphite that transforms at room temperature to diamond-like structures at pressures above 15 GPa. These structural transformations are reversible, and upon decompression graphite is recovered. More intriguing, however, are the phase transitions of silicon and germanium that crystallize at ambient conditions in the cubic diamond structure (Si-I or Ge-I) and transform to a body-centered tetragonal β-tin structure (Si-II or Ge-II) at 11.7 GPa and 9.7 GPa, respectively. Upon slow decompression, instead of returning to the most stable diamond structure, divergent transformation pathways lead to a body-centered cubic structure (BC8) for silicon or a simple tetragonal structure (ST12) for germanium. In this work, we report on a first-principles study of energetics and kinetics for the phase transition upon decompression in Si and Ge from the β-tin phase. We focus on the bond reconstruction processes that lead to the transitions from the β-tin phase toward the BC8, ST12 and diamond structure. In particular, we track the enthalpy change along various transformation pathways, examining not only the enthalpy difference of the end-structures, which is the commonly used criterion in evaluating phase transitions, but also the kinetic barriers along the pathways that provide crucial insights into relative competitiveness of different phase transition pathways. Using this approach, we have identified a body-centered tetragonal structure in I41/a symmetry that acts as a precursory state of the BC8 Si phase; this new structure has a small conversion barrier and a small lattice distortion from the β-tin phase via a double cell in-plane local-bond-rotation reconstruction mechanism. The kinetics of the pathway toward the BC8 Si is more favorable than that to the most stable diamond structure. We find that a similar phase transition is also viable in Ge under special conditions (e.g., rapid pressure release); however, a trinary cell local-bond-twisting reconstruction pathway toward the ST12 phase is energetically more favorable and kinetically competitive, making it the dominant pathway in Ge. The favorable kinetics is also the origin of the divergent pathway to the ST12 Ge phase over that to the diamond structure. Our results provide a comprehensive understanding of the experimental findings, underscoring the important role of kinetics in determining phase transformation in silicon and germanium. The detailed results have been published on Phys. Rev. Lett. 110, 165503 (2013). This study was supported by the NSFC of China (Grant No. 10974230, No. 11274356).
URL: http://link.aps.org/doi/10.1103/PhysRevLett.110.165503
URL: http://link.aps.org/doi/10.1103/PhysRevLett.110.165503
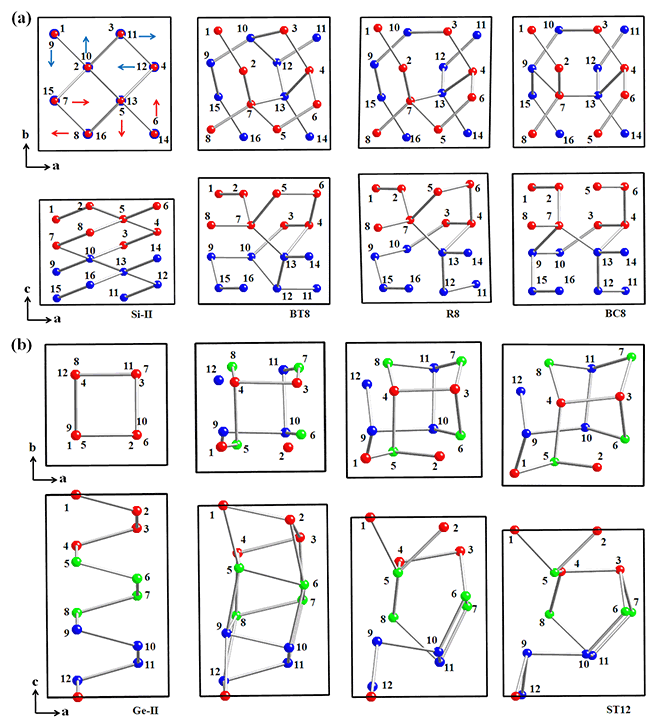 |
| Fig. 1: (a) Local-bond-rotation reconstruction process from Si-II to BC8 via BT8 and R8 phase. Si-II: a 16-atom tetragonal supercell in I41/amd symmetry containing two compressed diamond cells along the c[001] direction; BT8: a body-centered tetragonal structure in I41/a symmetry; R8: a distorted body-centered rhombohedral structure in R-3 symmetry; BC8: a body-centered-cubic structure in Ia-3 symmetry. (b) Local-bond-twisting reconstruction process from Si-II to ST12. Si-II: a 12-atom tetragonal supercell in I41/amd symmetry containing three conventional unit cells of Si-II along the c[001] direction; ST12 in P43212 symmetry. |
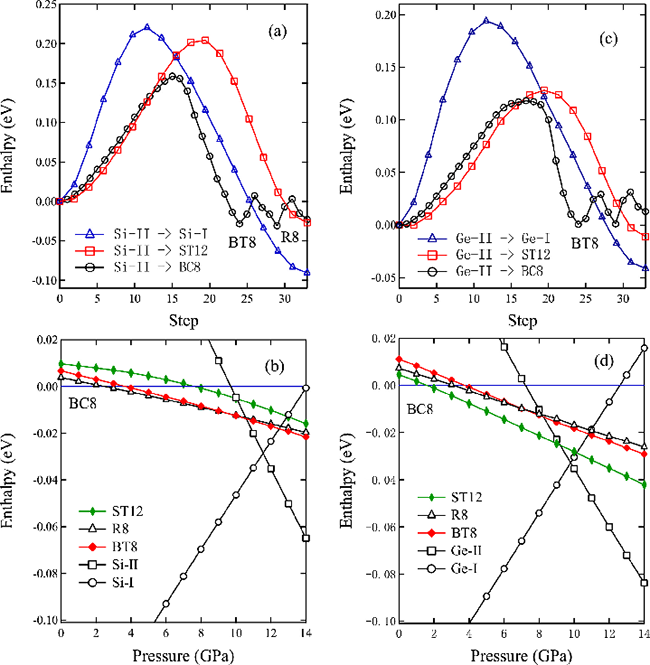 |
| Fig. 2: (color online) (a) Enthalpy versus transformation pathway Si-II → BT8 → R8 → BC8 and Si-II → ST12 in competition with Si-II → Si-I at 8 GPa. (b) Variation of the enthalpy with pressure for each of the Si phases under consideration, measured with respect to that of the BC8-Si phase. (c) Enthalpy versus transformation pathway Ge-II → BT8 → R8 → BC8 and Ge-II → ST12 in competition with Ge-II → Ge-I at 8 GPa. (d) Variation of the enthalpy with pressure for each of the Ge phases under consideration, measured with respect to that of the BC8-Ge phase. |


