5d cubic perovskite BaOsO3: ferromagnetic evolution over 3d to 5d series
Date:02-12-2013 Print
The perovskite-type transition metal (Tr) oxides are important from both viewpoints of fundamental study and practical applications. In the mostly studied d3 - d5 oxides, the octahedral TrO6 distortion and tilting will significantly affect the features of d electrons. A distortion-free perovskite series covering 3d to 5d oxides have therefore always been desired. The recent synthesis of 3d4 cubic BaFeO3 and 4d4 BaRuO3 enabled a study on d electrons from 3d to 4d, whereas the route to 5d electrons is blocked up due to the lack of a 5d4 cubic perovskite oxide.
Recently, SHI Youguo, an associate professor in WANG Nanlin’s group in Institute Of Physics, Chinese Academy of Sciences, through the collaboration with NIMS of Japan, reported the discovery of a cubic 5d oxide BaOsO3 (Fig. 1), an exact compound in continuation of the 3d4 to 5d4 cubic perovskite-type oxides free from octahedral TrO6 distortion. Unlike that cubic BaFeO3 and BaRuO3 show ferromagnetic transitions, the cubic BaOsO3 remains paramagnetic over the measured temperature range of 2 - 400 K.
However, compared with the Pauli paramagnetism in the first-principle calculations, the temperature independent term estimated from the measured magnetic susceptibility is much larger, indicating a significantly enhanced paramagnetism. Moreover, the density of states (DOS) at the Fermi level (EF) estimated from specific heat is also much larger than calculations. It looks like that cubic BaOsO3 is a ferromagnetic rather than a paramagnetic metal. A possible explanation is that, 5d4 BaOsO3 may not satisfy the Stoner criterion due to the significantly extended 5d valence orbitals that can reduced the DOS at EF as compared with those of 3d4 BaFeO3 and 4d4 BaRuO3, or caused by the remarkably reduced electronic correlation strength.
The magnetism evolution from 3d to 5d oxides has a tight relation with the structure, for example, the tolerance factor (left figure of Fig.2). Estimated from the effective magnetic moments from magnetic susceptibilities, the spin-orbital coupling in 3d4 BaFeO3 is negligible, whereas it plays a necessary role in 4d4 BaRuO3. In 5d4 BaOsO3, the spin-orbital coupling is enhanced, but it is hard to split the t2g band to have a gap as that occurs in Sr2IrO4 which has an antiferromagnetic Mott insulating ground state. The evolution of ferromagnetism with respect to some key physical parameters is indicated by the right figure. It is presumably that the spin-orbital coupling strength in cubic BaOsO3 is between that of 4d4 BaRuO3 and Sr2IrO4 and the BaOsO3 is on the phase boundary of a metal-insulator transition. However, to sufficiently explain the unusual properties of 5d4 BaOsO3, besides spin-orbital coupling, other physical parameters such as the on-site Coulomb repulsion and t2g band width should be taken into account.
This work was published in Journal of the American Chemical Society (135, 16507, 2013). The research was partially funded by the National Basic Research Program of China (973 program), and Chinese Academy of Sciences.
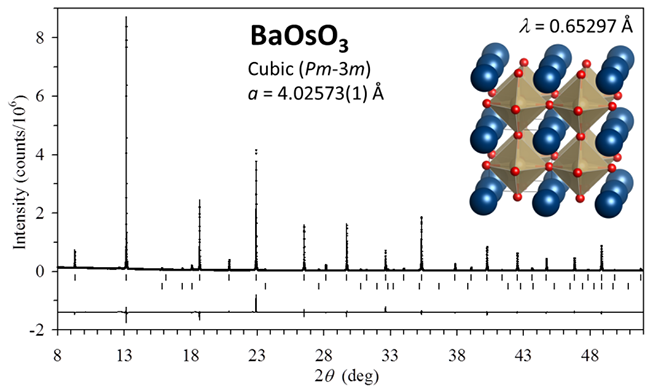 |
| Fig.1 Refined profile of the synchrotron XRD data for BaOsO3. Inset shows a structural view.(Image by IOP) |
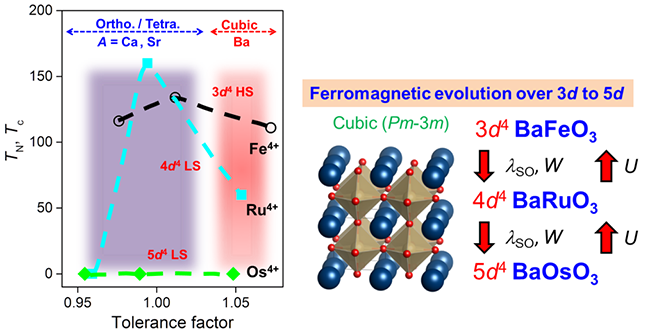 |
| Fig.2 (right) Magnetic transition temperatures as a function of tolerance factors for perovskite transition metal oxides;(left) Ferromagnetism evolution over 3d to 5d. (Image by IOP) |


