New Carbon Allotropes with Helical Chains in all-sp2 Bonding Networks
Date:27-03-2014 Print
Despite the existence of a large number of known carbon allotropes, the quest for new carbon structures has been a very active research field. The valence electrons of the carbon atom are capable of forming sp3-, sp2- and sp-hybridized states that support four basic types of single, double, triple, and aromatic carbon-carbon bonds, which are closely related to the bonding configurations in ethane, ethene, ethyne, and benzene-type hydrocarbon structures (see Fig. 1 for a comparative illustration). All carbon atoms in graphite are connected via the sp2 hybridization bonding with a bond angle of 120°and the graphite honeycomb lattice can be viewed as a planar molecule comprising benzene rings or 2-fold polyacetylene-like zigzag chains. The strong in-plane aromatic π-conjugation makes the two-dimensional three-connected (2D3C) graphite the most stable allotropic form of carbon. Diamond, which is related to polycyclic saturated hydrocarbon, is the second most stable allotrope of carbon with all the carbon atoms in a methane-like tetrahedral sp3 bonding, forming a very rigid 3D4C carbon network. Interestingly, the simplest 1D2C sp-carbyne has a polyyne-like alternating single and triple carbon-carbon bonds and it has been recently synthesized despite its rather high energy of 1 eV per atom above that of graphite. Beside carbyne, graphite and diamond, several other forms of carbon also have been synthesized; these include 0D3C fullerenes, 1D3C nanotubes, and 2D3C graphene. Conspicuously missing from this list of carbon structures is the long-sought 3D3C carbon in all-sp2 networks.
Under pressure, the highly crystalline varieties of graphite can be transformed to diamond via slipping, buckling, and cross-linking of the carbon sheets with sp2 → sp3 bonding transition. Accordingly we can construct the 3D3C carbon in all-sp2 networks via folding and cross-linking of the linear carbyne with sp → sp2 bonding state transition. Prof. WANG Jiantao from the Institute of Physics, Chinese Academy of Sciences and his collaborators identify by ab initio calculations a new type of 3D3C crystalline carbon in an all-sp2 network that comprises 3-fold, 4-fold, or 6-fold helical carbon chains. They found that the 3-fold structure (named cR6) has an 18-atom hexagonal unit cell in R-3m symmetry with six 3-fold helical chains as shown in Fig. 2(a), which topologically corresponds to the two-dimensional star lattice. It is shown as an (8,3)-net structure. Each carbon chain has three neighboring chains of complementary chirality with 3-fold screw axes running parallel to the z-axis. The six helical chains bond together forming a closed C12 armchair ring with alternating single and double carbon-carbon bonds, and two neighboring helical chains form a closed C8 zigzag ring with two double bonds as in 1,5-cyclooctadiene. The 4-fold structure (named cT8) has a 16-atom body-centered tetragonal unit cell in I41/amd symmetry with four 4-fold helical chains as shown Fig. 2(b), which topologically corresponds to the two-dimensional square lattice. It is also shown as an (8,3)-net structure. Each chain in 4-fold carbon has four neighboring chains of opposite chirality with 4-fold screw axes running parallel to the zaxis. Four 4-fold chains form a closed C8 armchair ring with alternating single and double carbon-carbon bonds as in tub-shaped 1,3,5,7-cyclooctatetraene, and two neighboring chains of opposite chirality bond together forming a closed C10 zigzag ring with two ethene-type planarπ-conjugation. Moreover, the 6-fold structure (named rh6) has an 18-atom hexagonal unit cell in R-3m symmetry with three 6-fold helical chains as shown Fig. 3(a,b), which topologically corresponds to the two-dimensional graphene lattice. The right-handed (or left-handed) helical chains in rh6 carbon have an alternating sp2-type single and double carbon-carbon bond. Meanwhile, three right-handed (or left-handed) helical carbon chains bond together forming a zigzag benzene ring. Therefore, rh6 carbon also can be regarded as a twisted graphite in AA stacking consisting of three zigzag hexagonal carbon rings with a (3x3x1) superlattice of graphite as shown in Fig. 3(c). These newly identified 3D3C allotropic forms of carbon comprise helical chains with alternating single and double bonds, thus termed chiral carbine.
Phonon and electronic band calculations show that these new carbon phases are dynamically stable and are semiconductor with a considerable band gap of 2.95 eV, 2.41 eV, and 0.47 eV for 3-fold, 4-fold, and 6-fold carbine, respectively. Experimental x-ray diffraction (XRD) of a TNT/dissel oil detonation soot revealed the presence of a considerable amount of amorphous carbon and several crystalline phases: the strongest peak around 26o comes from the graphite (002) diffraction; a weak peak around 43.6o is attributed to the diamond (111) diffraction; a prominent sharp diffraction peak at 30o, however, cannot be assigned to any known carbon phase such as graphite, diamond or fullerenes [see Fig. 4(b)]. A similar sharp diffraction peak at 30o was also found in the chimney soot and the trinitrotoluene/cyclomethylenetrinitramine detonation nanoparticles. The high intensity and sharpness of this unexplained XRD peak suggests that a new carbon phase has been consistently produced in these experiments. On the other hand, as shown in Fig. 4(a), the simulated XRD patterns for graphite and diamond are consistent with the corresponding experimental XRD peaks. Most importantly, the main peak for rh6 carbon matches almost perfectly the XRD peak at 30o for the previously unexplained carbon structure discovered in detonation experiments. This result suggests that the possible presence of 6-fold rh6 phase in soot carbon as well as the ultrafine diamond, graphite, and amorphous. The present results solve the long-sought 3D3C all-sp2 carbon structures and may help design other covalent bonding networks.
This study was supported by the National Natural Science Foundation of China (Grants Nos. 11274356, 11274012 and 91021007).
CONTACT:
Prof. WANG Jiantao
Institute of Physics
Chinese Academy of Sciences
Email: wjt@iphy.ac.cn
 |
| Figure 1. A comparative illustration of basic bonding configurations in ethyne, ethene, ethane, and benzene-type hydrocarbons and those in pure carbon allotropes. The typical carbon-carbon bond lengths with distinct bonding configurations are 1.54 Å for C(sp3)–C(sp3) single bonds as in diamond and ethane; 1.46 Å for C(sp2)–C(sp2) single bonds as in 1,3-butadiene and 1.48 Å in 3-fold and 4-fold carbene; 1.40 Å for aromatic bonds as in benzene and 1.42 Å in graphite; 1.34 Å for C(sp2)=C(sp2) double bonds as in ethene, 3-fold and 4-fold carbene; and 1.20 Å for C(sp)≡C(sp) triple bonds as in ethyne and carbine. (Image by IOP) |
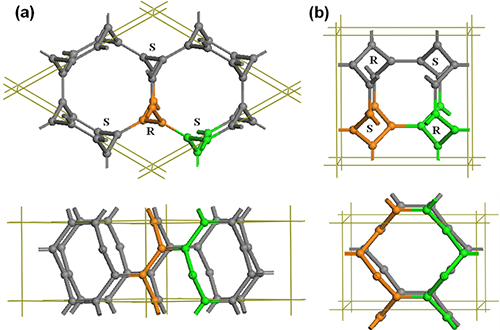 |
| Figure 2. Top and side views of the 3-fold and 4-fold chiral crystalline structures. (a) cR6 carbon in R-3m symmetry containing three 3-fold left-handed (S) and three 3-fold right-handed (R) chains. (b) cT8 carbon in I41/amd symmetry containing two 4-fold left-handed (S) and two 4-fold right-handed (R) chains. (Image by IOP) |
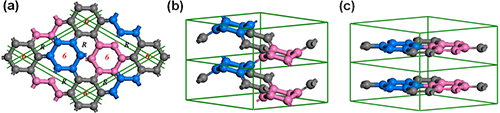 |
| Figure 3. Chiral crystalline modification of rh6 carbon in R-3m symmetry. Top (a) and side (b) view of rh6 carbon, which comprises three 6-fold helices or three zigzag benzene rings as its building blocks. It topologically corresponds to a (3x3x1) superlattice of graphite in AA stacking as shown in (c). (Image by IOP) |
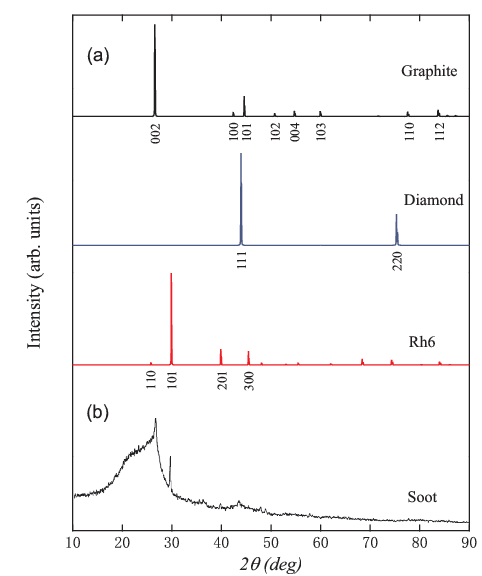 |
| Figure 4. X-ray diffraction (XRD) patterns. (a) Simulated XRD patterns for graphite, diamond, and 6-fold rh6 carbon. (b) Experimental XRD patterns for TNT/diesel oil detonation soot. X-ray wavelength is 1.5406 Å with a copper source. (Image by IOP) |
[1] New Carbon Allotropes with Helical Chains of Complementary Chirality Connected by Ethene-type π-Conjugation, J. T. Wang, C. F. Chen, and Y. Kawazoe, Sci. Rep. 3, 3077; DOI:10.1038/srep03077 (2013).
[2] A New Carbon Allotrope with Six-Fold Helical Chains in all-sp2 Bonding Networks, J. T. Wang, C. F. Chen, E. G. Wang, and Y. Kawazoe, Sci. Rep. 4, 4339; DOI:10.1038/srep04339 (2014).


