Magnetoelectric Effects Observed in a Perovskite Inorganic-organic Hybrid Material
Date:09-10-2014 Print
Conventional materials are usually divided into inorganic and organic materials. Hybrid inorganic-organic compounds represent a new class of solid-state materials. They could show an unprecedented variety of physical and chemical properties that are of great potential for many applications. Metal-organic frameworks (MOFs) consisting of metal ions connected by organic linkers are a prototype family of inorganic-organic materials. They have been known to show diverse chemical and physical properties arising from the organic-inorganic duality. Especially, dense MOFs with the ABX3 perovskite-like structure are of great interest because the different A and B components provide plenty room for adjusting the magnetic and electric properties in a simple crystalline structure.
The group led by Prof. SUN Young from Beijing National Laboratory for Condensed Matter Physics at the Institute of Physics, Chinese Academy of Sciences have been working on MOFs for several years and have achieved significant progress. They realized magnetic field control of electric polarization in a Mn-based MOF (Scientific Reports 2013, 3, 2024) and discovered resonant quantum tunneling of magnetization at low temperature in a Fe-based MOF (Phys. Rev. Lett. 2014, 112, 017202). Recently, they have made further progress in the study of multiferroics and magnetoelectric (ME) effects in perovskite MOFs.
Multiferroics refers to the coexistence of both electric and magnetic ordering in a single material. Based on the ME coupling effects in multiferroic materials, electric control of magnetism and magnetic manipulation of electric polarization can be achieved, which will provide additional freedom for developing novel functional devices. Since 2009, several groups reported the multiferroic behaviors in some perovskite MOFs. However, it is still a controversial question whether there could be ME coupling effects in multiferroic MOFs. In general, people have believed that there is no ME coupling in multiferroic MOFs because the magnetic ordering and ferroelectric ordering have different origins. Recently, SUN Young and coworkers developed an advanced ME effects measurement system and performed a precise study of the ME effects in single crystals of a perovskite MOF, [(CH3)2NH2]Fe(HCOO)3. This MOF has a spin-canted antiferromagnetic ordering below 20 K and a weak ferroelectricity below 164 K, representing a multiferroic system. A dielectric anomaly was observed just at the magnetic ordering temperature. The dielectric constant apparently depends on applied magnetic field in the multiferric state, evidencing the direct ME effect. Meanwhile, the converse ME effect, i.e., electric field control of magnetization, has also been observed at low temperatures. These experimental results present strong evidences for the ME coupling in multiferroic MOFs.
This work sheds new light on the physical mechanism of multiferroics in MOFs. It suggests that the ferroelectricity in MOFs is not simply due to the order-disorder transition of hydrogen bonding. Instead, a more complicated hybrid mechanism involving both A-site and B-site elements, as proposed by Dr. Stroppa and Picozzi based on first-principle calculations, may give rise to the ferroelectricity in perovskite MOFs. These studies have been published in Sci. Rep. 4, 6062 (2014) and Phys. Status Solidi RRL 8, 91 (2014). Since it is the first report of electric field control of magnetism in inorganic-organic materials, this work has been highlighted as research headline in Materials Views.
The project is supported by the National Science Foundation, the Ministry of Science and Technology of China, and the Chinese Academy of Science.
Link:
http://www.nature.com/srep/2014/140814/srep06062/full/srep06062.html
http://onlinelibrary.wiley.com/doi/10.1002/pssr.201308230/abstract
http://www.materialsviews.com/metal-organic-frameworks-for-electric-field-controlled-magnetism/
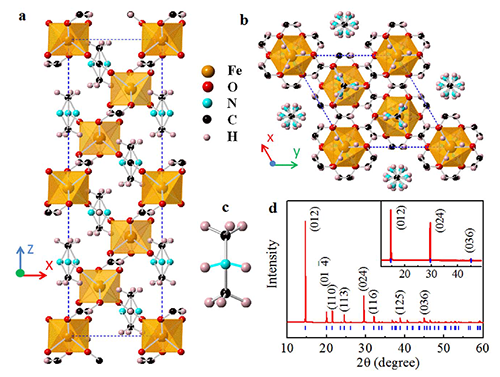 |
| Fig. 1. The crystal structure of [(CH3)2NH2]Fe(HCOO)3. |
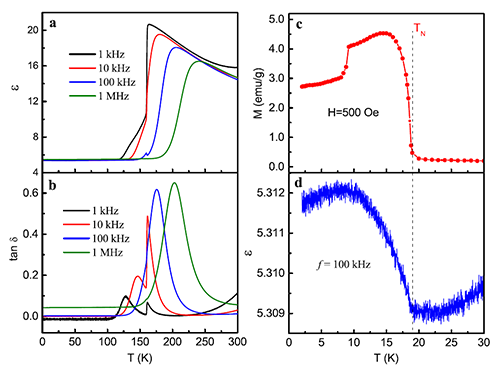 |
| Fig. 2. Coexistence of ferroelectric and magnetic ordering in (CH3)2NH2]Fe(HCOO)3. |
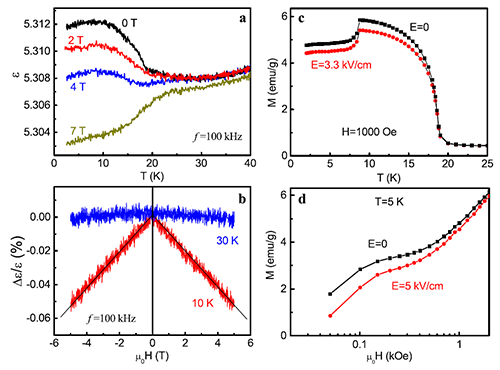 |
| Fig. 3. ME coupling in [(CH3)2NH2]Fe(HCOO)3. |


