Scientists Experimentally Realized Two-Dimensional Boron (borophene)
Date:01-04-2016 Print
Since the discovery of graphene, intensive interest has been focused on two dimensional (2D) materials, especially mono-elemental 2D materials which may possess novel physical properties beyond that of graphene. In the periodic table of elements (PTE), elements sitting around carbon are group IV elements such as silicon, germanium and tin, as well as its nearest neighbors such as boron and nitrogen. 2D graphene-like materials such as silicene, germanene, and stanene have recently been fabricated by scientists, but they are not stable in air. Black phosphorus is a stable allotrope of phosphor in nature, but single layer of black phosphorus is not stable in air and cannot be applied into nanodevice. Boron is the 5th element in PTE, and possesses sp2 hybridized orbitals similar as carbon, which favors the formation of low dimensional nanostructures such as nanotube and fullerene. Theoretical studies revealed that 2D boron (borophene) is also energetically stable. However, because of the extremely high melting point (over 2,000゜C) and low vapor pressure of boron, the experimental growth of borophene is very difficult, and the 2D form of boron has never been realized till now.
Recently, FENG Baojie and ZHONG Qing, the PhD students in Prof. WU Kehui and A/P CHEN Lan’s group from the Institute of Physics, Chinese Academy of Sciences, have experimentally realized 2D boron sheets (borophene) on Ag(111) surface for the first time. They employed high temperature crucible heated up to over 2000゜C by electron beam and evaporates boron onto the substrate directly in a homemade molecular-beam epitaxy system. They discovered two types of 2D B structures formed on Ag(111) using scanning tunneling microscopy. Boron atoms in 2D B arrange in triangle lattice with periodic arrangement of holes, which accords with previous theoretical predictions very well. They also found that the 2D B sheets bonds to the Ag(111) substrate only weakly, and it remains chemically stable against oxidization in air. Furthermore, in collaboration with ZHANG Jin, the PhD student in Prof. MENG Sheng’s group, and A/P LI Hui from Institute of Physics, they have performed first principle calculations on 2D B. The calculated physical properties of 2D B, including atomic structures, interactions between 2D B sheets and Ag(111) surface, and local density of states, agree well with experiments.
The experimental realization of such long expected 2D boron (borophene) structure is very exciting for the science society. Borophene is not only the newest mono-elemental 2D materials, but it also exhibits important physical properties, such as metallicity, flexible electronic property related to vacancy structure, stability against oxidization, and so on. This indicates the borophene could be a good candidate for device applications in the future.
This study entitled “Experimental Realization of Two-Dimensional Boron Sheets” was published on Nature Chemistry (DOI: 10.1038/nchem 2491).
This work was supported by the grants from the National Science Foundation, the Ministry of Science and Technology of China, and the Chinese Academy of Sciences.
Contact:
Institute of Physics
WU Kehui
Email:khwu@iphy.ac.cn
Key word:
Two dimensional materials; 2D boron; borophene; molecular beam epitaxy; scanning tunneling microscopy
Abstract:
Boron is the neighbor of carbon in the periodic table and has been theoretically predicted to form 2D sheet. Here, it is experimentally demonstrated that the two kinds of 2D boron sheets are formed on Ag(111) surface through molecule beam epitaxy. The sheets exhibit chemical stability against oxidation.
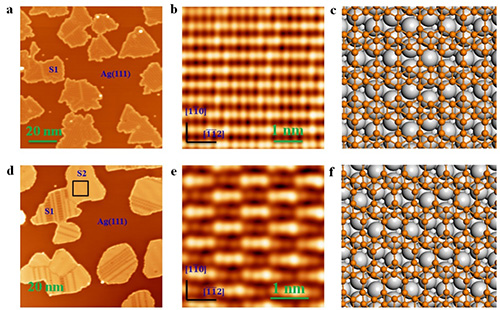 |
| Fig.1 (a,d) The scanning tunneling microscopy images of large area of 2D B sheets on Ag(111) surface. (b,e) The high resolution scanning tunneling microscopy images of two types of 2D B structures. (c,f) The atomic structure models of two 2D B structures. (Image fromNature Chemistry(DOI: 10.1038/nchem 2491)) |


