ZrB12 discovered as a “metallic diamond”
Date:22-11-2016 Print
Hard and superhard materials have brought substantial interest because of their wide technological applications in industry and the fundamental importance in condensed-matter physics. In conventional design of superhard materials such as diamond and cubic boron nitride, the pure covalent bonding network based on B-C-N-O systems is believed to provide both the low elastic compressibility and the high resistance to plastic deformation. Nevertheless, such pure localized covalence bonds also discourage electron transport in the B-C-N-O systems, making them large-gap insulators and severely limit their diverse applications such as hard coating, anvils of large volume press, etc. In this sense, employing high electrical conductivity to superhard materials to make “metallic diamond” becomes an urgent task for the research of superhard materials.
After careful exploration, Dr Xiaohui Yu, Dr Hui Li, Ma Teng, Xu Zheng, Prof. Changqing Jin, from Institute of Physics, Chinese Academy of Sciences, have found the high-boron-content TM borides, ZrB12, can possess both superhard mechanical properties and extremely high electrical conductivity.
Figure 1 shows the crystal structure of ZrB12 with space group of Fm3m refined from XRD pattern. The crystal structure of ZrB12 is tessellated by truncated octahedrons, where the B atoms occupy the vertices~forming the basic frameworks and the Zr atoms locate in the center of cages. Remarkably, these highly symmetrical polyhedrons formed by B-B bonds show no prominent shear path and the B-B bonds with bonding distances of 1.68 and 1.78 Å. Both of the above features predict the high resistance to the plastic deformation of ZrB12.
The Vickers hardness measurements were performed on the bulk polycrystalline and single crystal samples. As shown in Figure 2, the determined Vickers hardness of polycrystalline ZrB12 is ~ 45.0 (1.5) GPa under the load of 25 g and 40.2 (1.2) GPa under the load of 50 g, which exhibits the superhard property. When the load is increased from 25 g to 500 g, the measured Vickers hardness decreases and saturates at ~27.0 GPa, suggesting that ZrB12 is intrinsically in the regime of hard materials. Furthermore, the measurements of the Vickers hardness on single crystal ZrB12 show highly isotropic property on the {100}, {110}, {111} planes, which should be due to the high symmetric frameworks formed by B-B clusters.
In order to underline the physical origin of high hardness of ZrB12, the researchers have performed the first principle calculations. The anisotropic ideal shear strength of ZrB12 is accordingly derived from the calculated stress-strain relationships along different slip systems which are presented in Figure 3. It is seen that the shear strengths of ZrB12 is nearly isotropic within {111} planes with the minimum value of 34.5 GPa appearing along {111} [-1-12] deformation path, which is comparable to that B6O (38 GPa). Such results are in consistency with our experiments.
As shown in Figure 4, all the electric resistivity curves in different planes of ZrB12 show metallic behavior and a sharp superconducting transition at Tc= 5.5 K. In the case of ZrB12, the Zr atoms are constrained in the B-B truncated octahedrons, the distance between two nearest-neighbor Zr atoms (5.2 Å) is much larger than its Van der Waals diameter (3.2 Å), indicating the valence electrons cannot freely parade between Zr atoms. Surprisingly, the ZrB12 possesses such low electrical resistivity ~18 μΩ · cm (at RT), which is comparable to that of Pt~20 μΩ · cm (RT). Furthermore, the Seebeck coefficient of ZrB12 is only 2.0 μV/K at RT, which is also comparable to that of Cu and Pt, and could identify its excellent electrical transport behaviors as a metallic conductor.
To figure out the origin of the high electron conductivity of ZrB12, the projected electronic density of states (PDOS) is calculated and shown in Figure 5, which exhibits the bulk ZrB12 is metallic (Figure 5a). Twelve intersections between conduction bands and the Fermi level have been found on band structure throughout all the symmetry pathways in the Brillouin zone, as shown in Figure 5b. Therefore, based on the first principles calculation, we have confirmed the π-bonds between 2 icosahedral B12 motifs and Zr 4d orbitals can form highly dispersive bands at the Fermi level. Such conducting channels make ZrB12 a great conductor (Figure 5c-f).
This study entitled “Ultrastrong Boron Frameworks in ZrB12 : A Highway for Electron Conducting” was published on Advanced Materials. The study was supported by National Science Foundation of China.
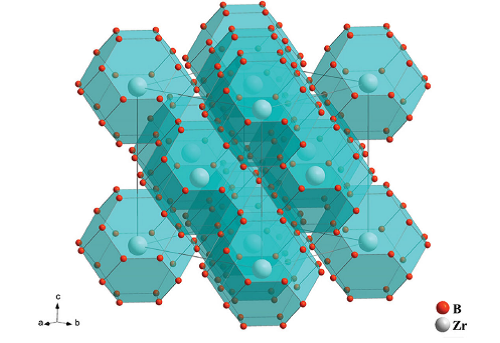 |
| Fig1:a polyhedral view of the crystal structure of orthorhombic ZrB12. |
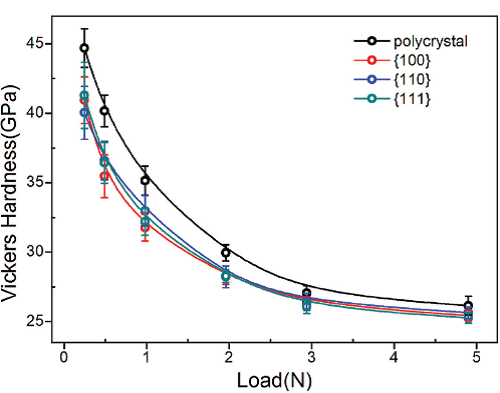 |
| Fig 2: Measured Vickers hardness as a function of applied loads from 25g to 500 g。 |
 |
| Fig 3:The calculated stress-strain relationships for ZrB12under various loading conditions |
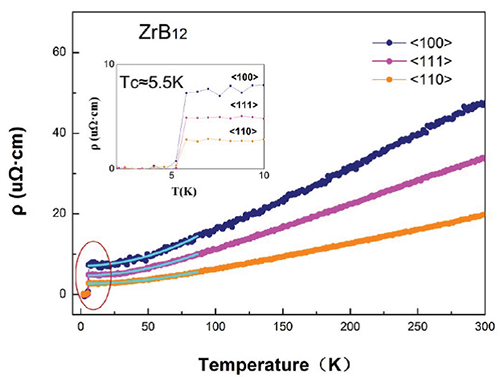 |
| Fig 4:electrical resistivity measurement of ZrB12from 300K to 2K. The inner picture shows the fitting of electrical resistivity from 6-60K。 |
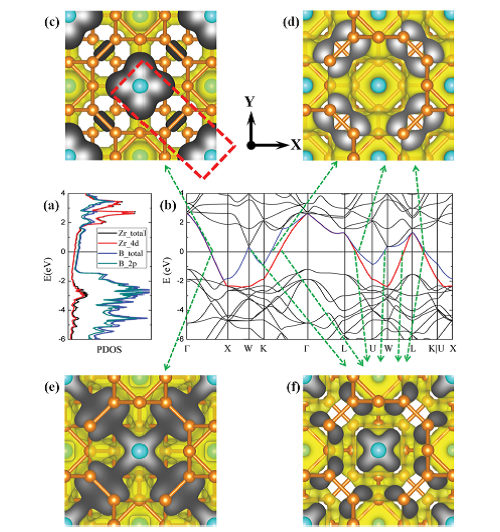 |
| Fig 5:(a) Projected density of states (PDOS) for ZrB12. (b) Band structures of ZrB12around the Fermi level. The red (band 21) and blue (band 22) lines denote to the two bands crossing the Fermi level, respectively. (c-f) The distributions of partial band decomposed chargedensity (isovalue = 0.0035 Å-3) at various k-points at the intersections between conduction bands and the Fermi level (directed by the green arrows). Color code: Zr (large blue ball), B (small orange ball), Charge density (yellow surface and gray scaled section). The atomic models and chargedenisty are visualized by VESTA3 package. The red rectangle in Fig. 1(c) denotes to the Zr-BB-Zr Bridge structure. |
Contact:
Institute of Physics
Yu Xiaohui
Email:yuxh@iphy.ac.cn
Key word:
Zirconium dodecaboride; boron frameworks; Superhard; ultralow electrical resistivity
Abstract:
ZrB12,with a high symmetrical cubic structure, possesses both high hardness~27.0 GPa and ultralow electrical resistivity~18 μΩ · cm at room temperature. Both superior conductivity and hardness of ZrB12 are associated with the extended B-B 3D covalent bonding network as it is not only favorable for achieving high hardness, but also provides conducting channels for transporting electrons.


