3D-disordered Cation Framework Stabilize the Oxygen Redox
Date:08-04-2019 Print
Li-ion batteries (LIBs) have been regarded as the technology of choice for energy storage fields. The development of large-scale energy storage requires higher energy density of LIB. Li-rich cathode oxides with ultrahigh capacity have the greatest application potential for high-energy-density LIBs. Lattice oxygen redox (l-OR) is essential for achieving the high capacity in this system materials. However, till now, the understanding of l-OR chemistry remains elusive, and a critical question is the structural effect on the stability of l-OR.
Neutron pair distribution function (nPDF), capable of emphasizing the oxygen atomic scattering, is a powerful tool to detect both the local and average oxygen lattice structure evolution upon the l-OR. Combing the nPDF method with scanning transmission electron microscopy (STEM), recently ZHAO Enyue and Li Qinghao in Prof. WANG Fangwei and Prof. YU Xiqian’s groups from Institute of Physics, Chinese Academy of Sciences (IOP, CAS) cooperated with Prof. GU Lin (IOP, CAS) and Prof. YANG Wanli (Lawrence Berkeley National Laboratory Berkeley, USA) have revealed the coupling between l-OR and structure dimensionality.
The researchers provided the direct experiment evidence for the existence of l-OR via mapping of resonant inelastic X-ray scattering. Meanwhile, nPDF and STEM suggested that the oxygen lattice structure evolution upon the l-OR in 3D-disordered Li-rich oxides is stable, contrasting the distorted oxygen lattice in 2D/3D-ordered Li-rich oxides. It was indicated that the differentiated structure variation is directly correlated with the structure dimensionality. The l-OR usually occur on the lattice O2- ions with unhybridized O2p states. To reduce the energy, the distance between two oxidized O2- ions would shrink (i.e., oxygen lattice distortion). This oxygen lattice distortion occurs only if two unhybridized O2p orbitals are coplanar. In 3D-disordered Li-rich oxides, the formation probability of coplanar unhybridized O 2p orbitals is very low. Thus, a stable oxygen lattice was observed in 3D-disordered Li-rich oxides, which in turn favor the stable l-OR.
This work shows that the structure dimensionality can be tuned to promote the stable l-OR with robust oxygen lattice in Li-rich cathode oxides, providing new insights for both fundamental researches and practical developments of high-energy-density cathodes relying on stable l-OR chemistry.
This study entitled "Stabilizing Oxygen Lattice and Reversible Oxygen Redox Chemistry through Structural Dimensionality in Li-rich Cathode Oxides" was published on Angew. Chem. Int. Ed.
The study was supported by the National Key R&D Program of China, National Science Foundation of China, Songshan Lake Materials Laboratory and China Spallation Neutron Source.
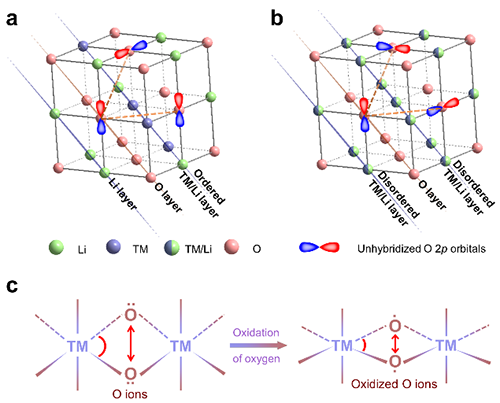 |
| Fig.1 Structure schematic for Li-rcih oxides. (Image by Wiley-VCH) |
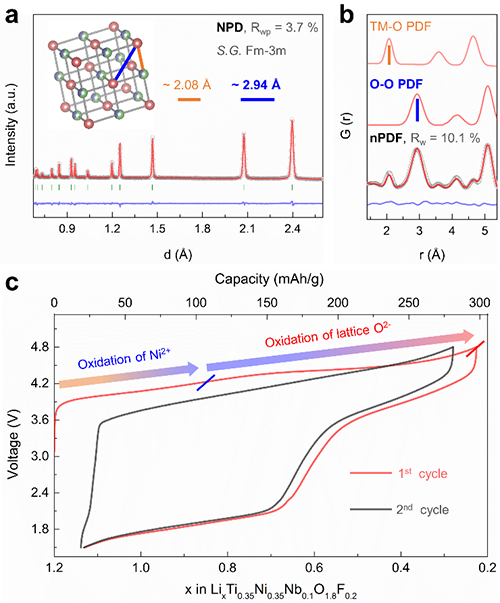 |
| Fig.2 Neutron powder diffraction, neutron pair distribution and electrochemical data for Li1.2Ti0.35Ni0.35Nb0.1O1.8F0.2. (Image by Wiley-VCH) |
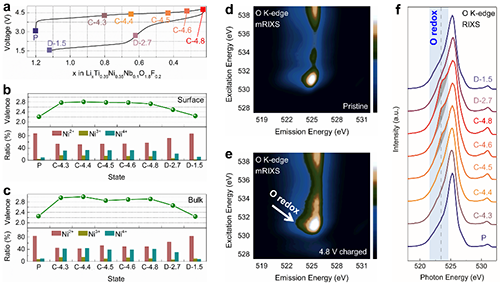 |
| Fig.3 Charge compensation process for Li1.2Ti0.35Ni0.35Nb0.1O1.8F0.2. (Image by Wiley-VCH) |
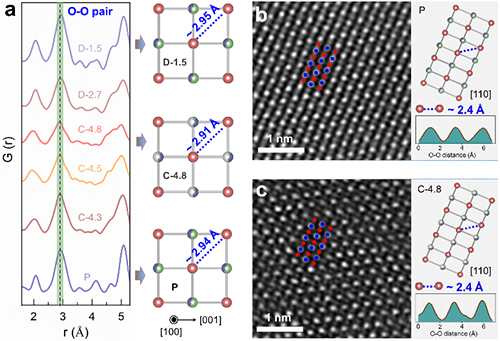 |
| Fig. 4 Oxygen lattice structure evolution upon the l-OR. (Image by Wiley-VCH) |
Contact:
Institute of Physics
WANG Fangwei
Email:fwwang@iphy.ac.cn
Key word:
Li-ion battery; Li-rich cathodes; lattice oxygen redox; structure dimensionality.
Abstract:
Lattice oxygen redox (l-OR) is essential for achieving the ultrahigh capacity in Li-rich cathode oxides. However, the understanding of l-OR chemistry remains elusive. Herein, the coupling between l-OR and structure dimensionality is researched. We reveal that the 3D-disordered cation framework can be used to stabilize the oxygen lattice upon the l-OR. This work highlights the role of structure dimensionality in tuning the stability of oxygen lattice structure involving l-OR.


