Protein Dynamical Structure Regulates Photoprotection in Light Harvesting Complex II
Date:24-06-2020 Print
The photosynthetic systems of green plants have developed a dual function of efficient light collection under low light intensity and photoprotection under intense sunshine to prevent the oxidative damage of reaction centers. Whereas it is widely accepted that the such a dual function is mainly realized at the most abundant light-harvesting complex II (LHCII) in response to the photo-induced pH change at lumenal side, a long-lasting puzzle remains that how this photosynthetic protein switches between these two opposite functions via fast structural change in adaption to changes of the environmental condition? Obviously, the ultimate answer to this problem would be instructive to the molecular breeding and genetic engineering of plants with much higher photosynthetic efficiency.
In their integrative study of protein dynamical structural change combining time-resolved spectroscopy, molecular dynamics simulations, and density functional theory calculations, the researchers reported that aggregated LHCII trimer acts as a molecular machine driven by elevated temperature and drop of pH induced by the photo-irradiation as signals for the environmental stress. These signals trigger fast protein allosteric motions and shorten the inter-pigment separation between lutein and chlorophyll pairs, thereby dissipating the excess excitation energy transfer from Chla to the dark state of lutein as heat. This mechanism reconciles the macroscopic environmental factors and microscopic protein structural rearrangement at atomic level, and elucidates the causality exhibited in the plants as to the environmental stress, providing a novel insight to the above question, one of the core questions in the photosynthesis research.
Explicitly, elevation in temperature or drop in pH leads to the fast formation of two nascent helix segments at the ends of helix E and D respectively, the membrane insertion of helices E and D pushes the scissor arms constituted by a crossed pair of membrane helices A and B to a more opened position, which switches off the excited-state energy transfer pathway, leaving the system in a photoprotection state. While under low sun illumination and higher pH, the conformation undergoes a reverse change, and molecular machine switches back to its energy transfer pathway, setting the system back to its light-collection and energy transfer state.
This study entitled "Dynamical allosteric regulation of photoprotection in light harvesting complex II" was published on Science China Chemstry
The study was supported by the National Science Foundation, the Chinese Academy of Sciences and grants from US.
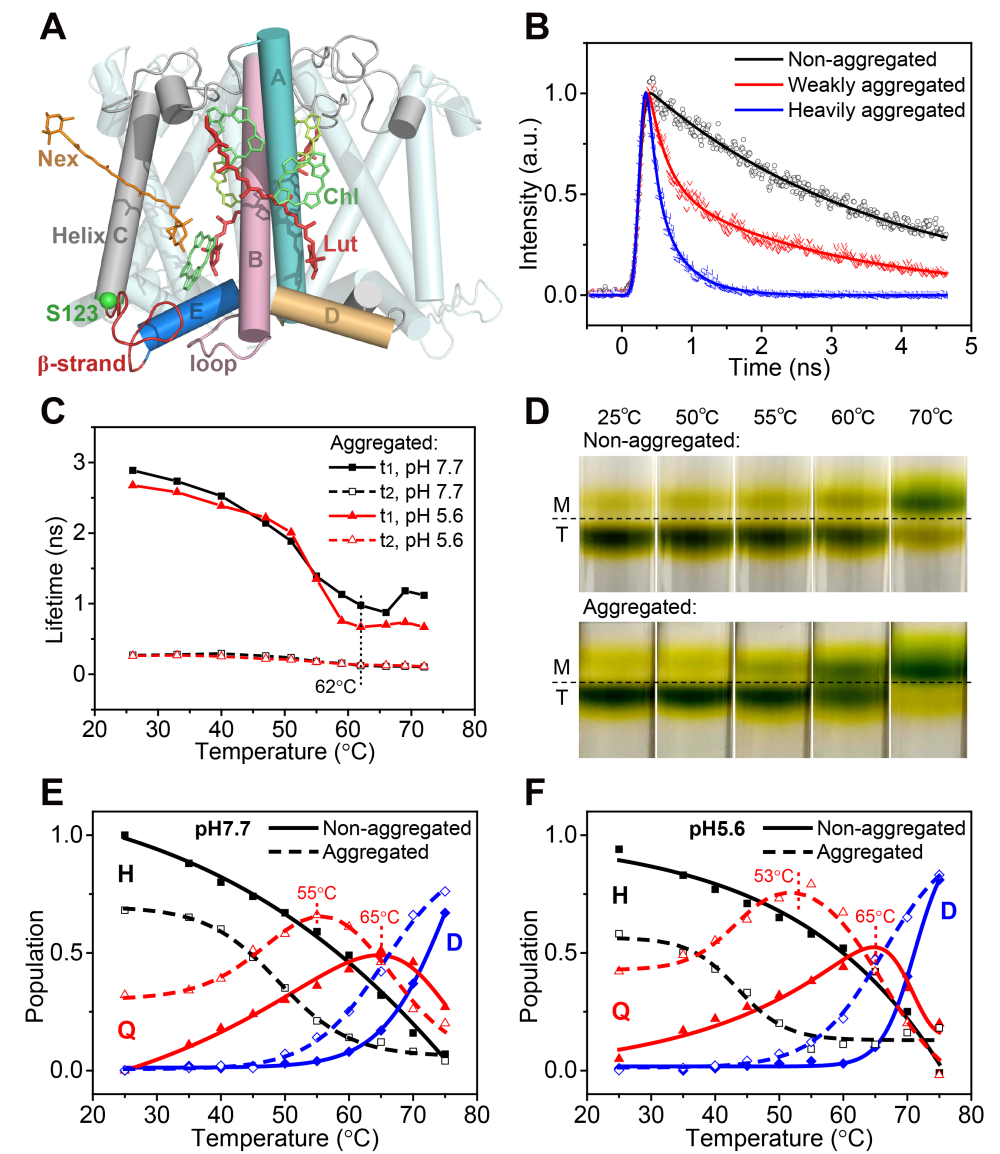
Fig.1 Figure 1. Crystal Structure and the Fluorescence Decay Kinetics of LHCII Trimer. H: Light-harvesting conformer; Q: quenching conformer, and D: denatured conformer. (Image by Institute of Physics)
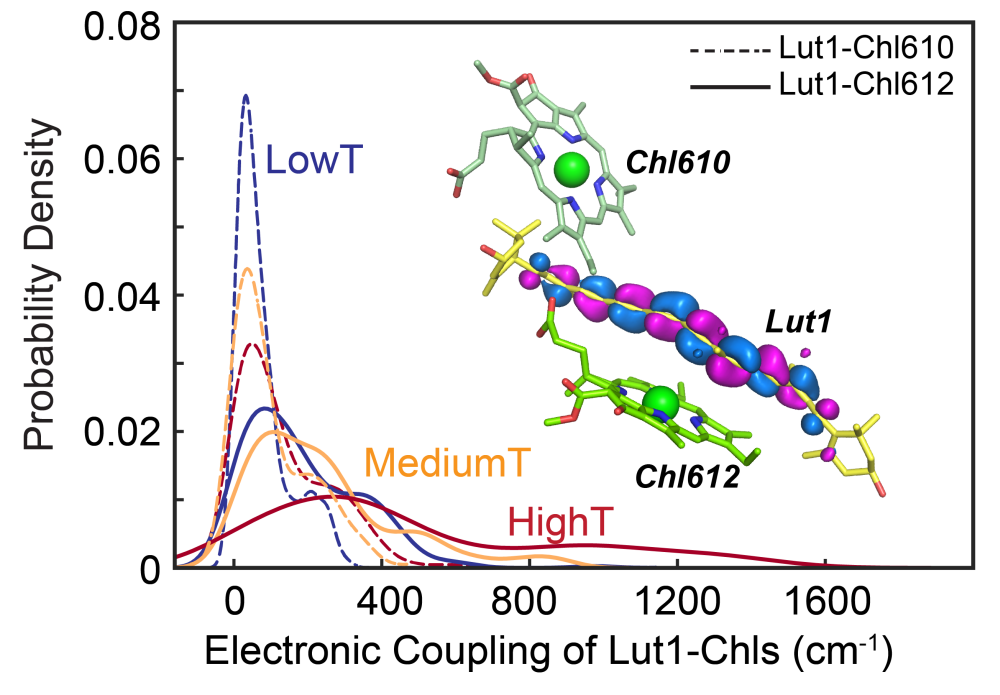
Fig.2 Temperature-induced Electronic Coupling between Lut1 and Chl and Configurational Twist of Lutein for LHCII Trimer. (Image by Institute of Physics)

Fig.3 Dynamical and Allosteric Regulation Mechanism for Light-Harvesting and Photoprotection of LHCII trimer. (Image by Institute of Physics)
Contact:
Institute of Physics
WENG Yuxiang
Email:yxweng@iphy.ac.cn
Key word:
LHCII photoprotection, conformational dynamics and allostery, protein switch, Temperature-jump, MD Simulations
Abstract:
Major light-harvesting complex of photosystem II (LHCII) plays a dual role of light-harvesting and excited energy dissipation. However, the molecular origin of the protein dynamics at the atomic level is still unknown. This work reveals that the dynamical allosteric conformation change induced by thermmal and acidity leads to close contacts between the carotenoid lutein 1 and photo-activated chlorophyll pigment 612, switching from light-harvesting state to the fluorescence quenching state fror energy dissipation .


