Spin pinning effect in interface for enhanced water oxidation
Date:12-07-2021 Print
Solar water splitting, water electrolysis, and regenerative fuel cells are the major methods to produce "green hydrogen energy", which is significant to the realization of the sustainable energy strategy. The sluggish kinetics of oxygen evolution reaction (OER) is a major cause for the low of efficiency in the process of realizing the hydrogen energy infrastructure. Exploring better catalysts for OER is now intensively studied in recent years. For the cost-effective non-precious 3d-transition metal oxides (TMOs), most of research works focus on the tuning of the binding strength between the metal and the oxygen species to optimize the free energy of the intermediate resulting a low OER overpotential in the thermaldynamics. The surface magnetic structures of the catalyst and the tuning spin-dependent kinetics barrier in OER have become increasingly attractive. The suitable surface magnetic configuration of the catalyst and the kinetics mechanism of the electron transfer in OER under a magnetic field becomes the key problems in the OER research field.
In recent years, Assoc. Prof. Haitao Yang from Group N04 in Institute of Physics of the Chinese Academy of Sciences and Prof. Zhichuan Xu from Nanyang Technological University have been cooperating together to study the spin related effect on the electro-catalysis. they reported that the OER can be enhanced by using ferromagnetic ordered catalysts as the spin polarizer for spin selection under a constant magnetic field. This work will be helpful for further development of magnetic field assisted OER-enhancing strategy and related catalysts. (Nature Communications 12,2608(2021)).
Recently, they reported that a high-performing oxideFM/oxyhydroxide system where the spin pinning has been realized intrinsically in Co oxyhydroxide layer on FM CoxFe3-xO4 substrates (oxideFM/oxyhydroxide). Under spin pinning effect, the spins can be further aligned after a short-time magnetization under magnetic field, which further increases the OER activity. The spin pinning effect provides them with a great potential for boosting spin-dependent kinetics to further enhance OER performance. They have achieved a stable oxideFM/oxyhydroxide configuration of CoxFe3-xO4/Co(Fe)OxHy with a controllable reconstruction by the sulfurization of the CoxFe3-xO4 oxides under OER condition (Figure 1). At the interface, FM magnetic domains in CoxFe3-xO4 with highly aligned spins can result in a strong pinning of the spins in oxyhydroxide layer, for which the reconstructed oxyhydroxide exhibits higher intrinsic OER activity than directly prepared Co(Fe) oxyhydroxides by ~1 order of magnitude。The magnetic hysteresis loops of the reconstructed and pristine CoFe2O4 shows an exchange bias effect in the CoxFe3-xO4/Co(Fe)OxHy formed after reconstruction,which originates from uncompensated interfacial spins that are pinned in the oxyhydroxide layer. After magnetization, the OER enhancement was notable only if the spin pinning had been established with FM substrates, and its effect can be turned OFF by demagnetization (Figure 2). This implies that the spin pinning effect is involved in the rate-determining step (RDS) of OER, and the RDS also believed to be spin-related. Under spin pinning, the 2p electrons in reactant oxygens with specific spin state (spin up or spin down) can transfer through catalysts during OER process, which creates oxygens with parallel spin alignment and thus promoting the production of triplet oxygen (Figure 3). Besides, it is also believed that the involvement of the oxyl radicals with more unpaired non-bonding p electrons in reconstructed oxyhydroxide is critical for the spin polarization in OER. As the oxyl radicals are created at the first dehydrogenation step, the spin polarization of oxygens can be facilitated accordingly under spin pinning, which reduces the barrier for the subsequent -O-O- coupling (RDS); otherwise to reach oxygen radicals with parallel spin alignment will cause additional barrier before O2 turnover (Figure 4). This finding showcase that the usage of spin pinning effect is a promising way of engineering QSEI in non-FM catalysts to improve catalysis. This work will be helpful for boosting spin-dependent kinetics to further enhance OER performance.
The results have been published on line in Nature Communications (Nat. Commun. 12, 2608 (2021)). Tianze Wu and Xiao Ren contributed equally to this work. Haitao Yang and Zhichuan Xu are corresponding authors. The work at IOP is supported by grants from the National Natural Science Foundation of China (11274370 and 51471185), the National Key Research and Development Projects of China (2016YFJC20013, and 2018YFA0305800), and Authors in Singapore thank the support from the Singapore Ministry of Education Tier 2 Grants (MOE2017-T2-1-009 and MOE2018-T2-2–027).
Link of the article:
https://www.nature.com/articles/s41467-021-23896-1
https://www.nature.com/articles/s41467-021-22865-y
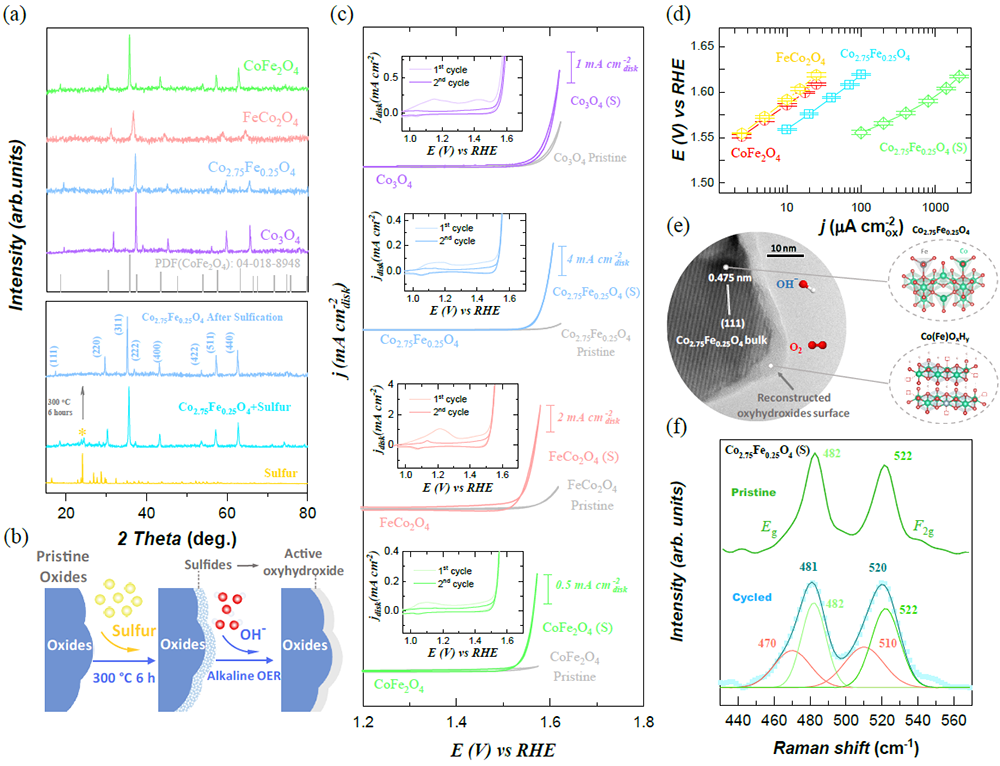
Fig. 1 Controllable surface reconstruction on Co3-xFexO4 spinel oxides for OER. a The powder X-ray diffraction (XRD) patterns of as-synthesized Co3-xFexO4 (x=0–2.0) (top) and Co2.75Fe0.25O4 as selected example before and after sulfurization (bottom). b The schematic diagram of a controllable reconstruction on oxides by controlling the sulfurization. The sulfurized products are denoted as Co3-xFexO4 (s). c The cyclic voltammetry (CV) curves of Co3-xFexO4 (x=0–2.0) in O2-saturated 1 M KOH with a scan rate of 10 mVs-1. Inset is the first and second CVs of sulfurized oxides (noted as Co3-xFexO4 (s)). d The Tafel plots of the OER specific activity of Co2.75Fe0.25O4 (s) versus pristine Co3-xFexO4 oxides. The plots are given after oxide surface area normalization, capacitance correction, and iR correction. The error bars represent the standard deviation from three independent measurements. e The high-resolution transmission electron microscope (HRTEM) image of Co2.75Fe0.25O4 (s) after reconstruction (i.e., Co2.75Fe0.25O4/Co(Fe)OxHy). The HRTEM sample is from the electrode that was cycled without adding carbon. The bulk Co2.75Fe0.25O4 oxide is covered by an amorphous (oxy)hydroxide layer with thickness of ~4 nm. f The Raman spectra of Co2.75Fe0.25O4 (s) before and after operating under OER. The peaks at Raman shift of 482 and 522 cm-1 are assigned to Eg and F2g mode in Co2.75Fe0.25O4 spinel. The broad peaks at Raman shift of 470 and 510 cm-1 are resulted by the bending and stretching of O-Co-O in amorphous Co(Fe)OxHy.
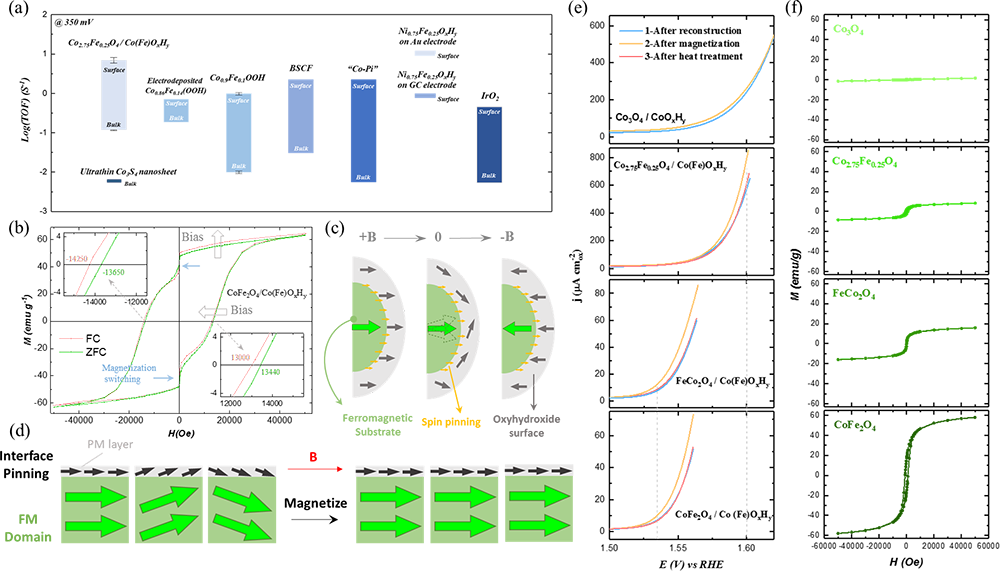
Fig. 2 OER enhancement by spin pinning in Co3-xFexO4/Co(Fe)OxHy under magnetic field. a Turnover frequency (TOF) values of reconstructed Co2.75Fe0.25O4(s) (i.e., Co2.75Fe0.25O4/Co(Fe)OxHy), electrodeposited Co0.86Fe0.14(OOH), Co0.9Fe0.1OOH, Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF) film, electrodeposited cobalt hydroxide (Co-Pi), Ni0.75Fe0.25OxHy NPs on Au electrode, electrodeposited Ni0.75Fe0.25OxHy on glassy carbon (GC) electrode, IrO2 (in acid), and ultrathin Co3S4 nanosheet. The TOF bulk and TOF surface of some catalysts present the lower and upper limits of the estimated TOF for fair comparison. The methods for TOF evaluation are given in the "Methods" section. The error bars represent the standard deviation from three independent measurements. b The magnetic hysteresis loops of CoFe2O4 (s) after 10 OER CVs (i.e., CoFe2O4/Co(Fe)OxHy) under both fieldcooled (FC) mode and zero-field-cooled (ZFC) mode. The inset provides enlarged panels showing the loops that intersect with y = 0 axis. c The schematics of the exchange bias under switching magnetic field, which is closely associated with the spin pinning effect at the interface of ferromagnetic substrate/oxyhydroxide. d The schematic illustration of the spin pinning effect at the interface between ferromagnetic (FM) magnetic domains and the thin paramagnetic (PM) oxyhydroxide layer, and the spins in the PM oxyhydroxide layer can be aligned more under magnetization. e Linear sweep voltammetry (LSV) of Co3-xFexO4 (s) after reconstruction in 1 M KOH following the procedures: (1) after reconstruction (10 CV cycles in 1 M KOH, light blue), (2) after magnetization under 0.5 T for 15 min and the removal of the magnetic field (yellow), and (3) after the post-treatment at 120 °C for 1 min (pinkish). The gray dash lines denote the OER potential where the current density has been improved by 20% compared to that before magnetization. The error bars represent the standard deviation from three independent measurements given in Supplementary Fig. 17. f Magnetic hysteresis loops of Co3-xFexO4 oxides.
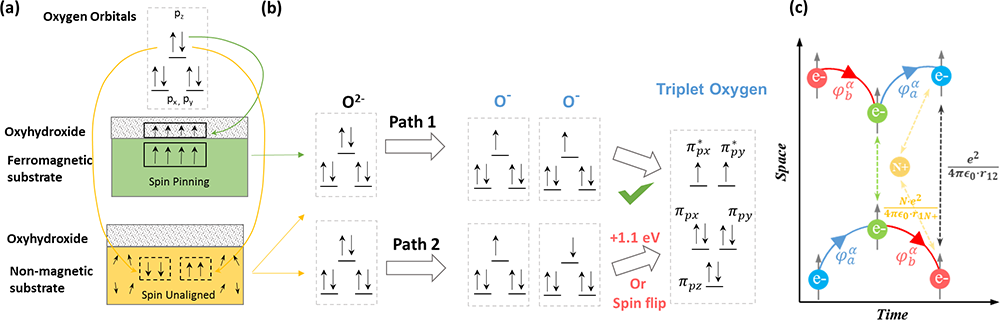
Fig. 3 Spin pinning effect for triplet oxygen evolution on the oxyhydroxide. a The spin-electron transfer from singlet oxygen (OH-, H2O) to Co3-xFexO4/ Co(Fe)OxHy with and without spin pinning effect. b The triplet oxygen production by two oxygen radicals in parallel/opposite spin alignments. c The QSEI mechanism in a space–time Feynman diagrams. Two electrons with the same spin approach, in time from the left side, to avoid the increase of the Coulomb repulsions; the electrons exchange their orbitals (momentum) to effectively keep them apart. φ is the wavefunction of the orbitals (momentum) of spin electrons. The electronic repulsion between spin electrons is given as \(\frac{e^{2}}{4 \pi e_{0} r_{12}}\) and electron–nuclei Coulomb attraction is given as \(\frac{N \cdot e^{2}}{4 \pi e_{0} r_{1 N^{+}}}\).
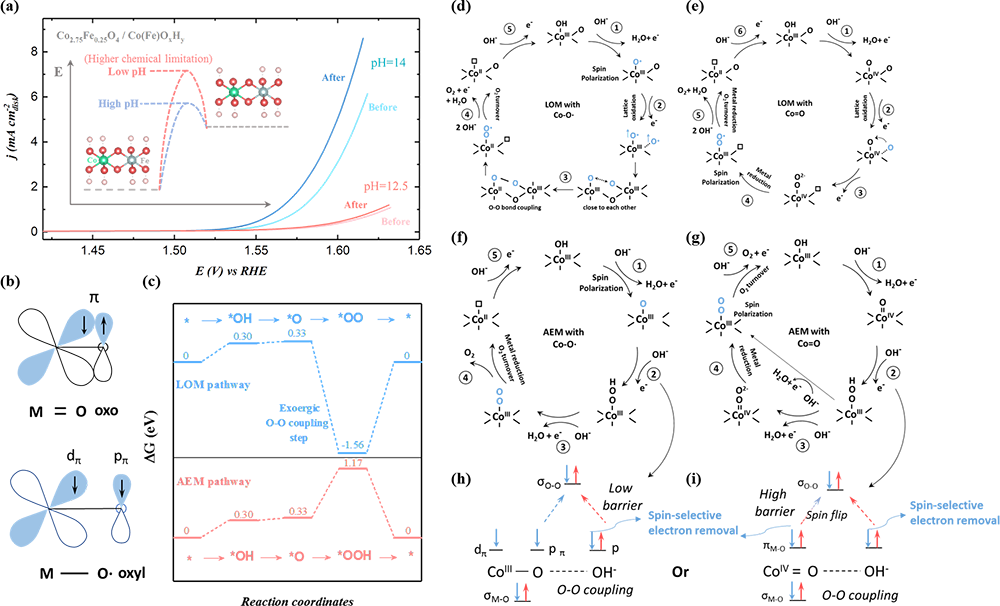
Fig. 4 Active lattice oxygen participation for producing triplet oxygen. a The linear sweep voltammetry (LSV) of the reconstructed Co2.75Fe0.25O4 (s) (cycled in 1 M KOH for 10 cycles to evolved Co2.75Fe0.25O4/Co(Fe)OxHy) before and after magnetization in magnetic field of 0.5 T for 15 min under pH of 12.5 and 14. The inset is a schematic illustration of dehydrogenation under different pH for generating negatively charged oxygen on Co site. b The spin configurations of M = O oxo and M-O·oxyl species. c The free energy diagram of OER under AEM and LOM pathways at 1.23 V (vs. RHE) on β-Co(OOH) (001) slabs toward triplet oxygen production. d, e The LOM OER pathway for the spin polarization mechanism d with Co-O·oxyl radical and e with Co=O oxo species. The early spin polarization mechanism is closely associated with the creation of oxyl radicals. f, g The adsorbate evolution mechanism (AEM) OER pathway for the spin polarization mechanism f with Co-O·oxyl radical and f with Co=O oxo species. h, i The spin-related O-O coupling process under AEM h with Co-O·oxyl radical and i with Co=O oxo species.


