OSTα/β transport mechanisms revealed by cryo-EM
Date:27-01-2026 Print
Bile acids (BAs) are multifunctional amphipathic surfactants. BAs act as key regulators for the digestion, absorption, and excretion of lipids, and serve as the modulation of critical signaling pathways involved in metabolism, inflammation, and liver function. The organic solute transporter α/β (OSTα/β) plays a central role in maintaining systemic BA homeostasis. OSTα/β facilitates the transport of BAs from enterocytes to the portal circulation, a key process for maintaining BA homeostasis. Dysregulation of OSTα/β is implicated in the pathogenesis of cholestasis, pruritus, and nonalcoholic steatohepatitis (NASH).
However, despite its physiological importance, the precise molecular mechanism of OSTα/β transport of BAs remains unknown. Recently, Prof. Jiang Daohua's group determined the high-resolution cryo-electron microscopy (cryo-EM) structures of human OSTα/β, which reveals its novel assembly architecture and the mechanism underlying bile acid transport.
The study shows that OSTα/β assembles into a unique dimer-of-heterodimers complex consisting of two OSTα and two OSTβ subunits. Extensive intermolecular interactions between OSTα-OSTα and OSTα-OSTβ subunits confer high structural stability to the assembly (Fig. 1). The key positive K191 of the BAs binding pocket engages the negatively charged moiety of the BAs, thereby facilitating BAs transport. Furthermore, molecular dynamics (MD) simulations revealed that the BA undergoes a "head-down" to "head-up" flip across the membrane during BAs translocation, while the TM core domains of OSTα/β remain relatively stable throughout this process (Fig. 2).
This study elucidates the overall architecture of the human OSTα/β complex and its novel BAs bidirectional transport mechanism, which is distinct from the canonical alternating-access model of most transporters. These results will help researchers to understand the structure-function relationships of OSTα/β, and will stimulate further investigations of OSTα/β-associated drug disposition, potentially leading to the development of therapeutics.
This study entitled "Structure and mechanism of the human bile acid transporter OSTα–OSTβ" was published on Nature. Link for the article:https://www.
This study was supported by the National Natural Science Foundation of China, The Chinese Academy of Sciences and The Institute of Physics.
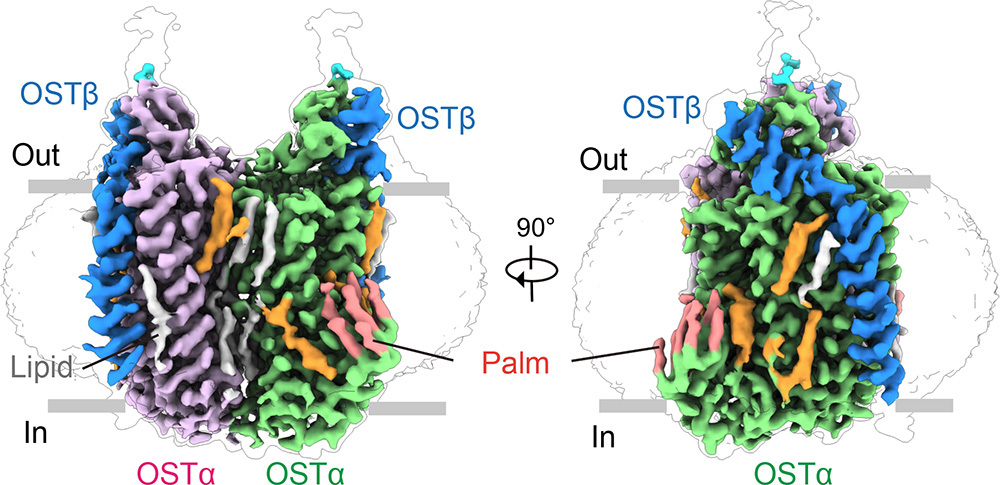
Fig.1 Cryo-EM structure of human OSTα/β (Image from Institute of Physics, SM10)
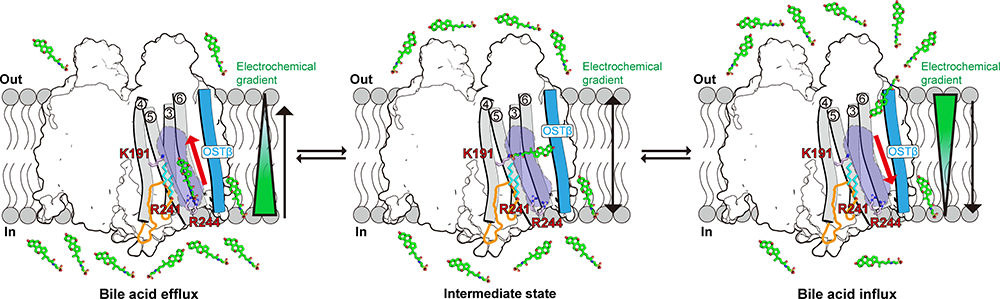
Fig.2 The bidirectional BA transport model of OSTα/β (Image from Institute of Physics, SM10)
Contact:
Institute of Physics, Laboratory of Soft Matter Physics.
Jiang Daohua
Email: jiangdh@iphy.ac.cn.
Key words:
OSTα-OSTβ; Cryo-EM; Transport mechanism; Bile acids homeostasis.
Abstract:
Bile acids (BAs) are crucial amphipathic surfactants that function as multifaceted regulators in various physiological processes, including nutrient absorption and distribution, lipid metabolism and inflammation. The human organic solute transporter (OSTα-OSTβ, hereafter referred to as OSTα/β) is a BA transporter that has a key role in the secretion and distribution of BAs. Pathogenic mutations in OSTα/β have been associated with cholestasis. Despite the functional importance of OSTα/β in BA homeostasis, the stoichiometry and assembly of the complex and the molecular mechanism that underlies BA transport by OSTα/β remain unknown. Here we present cryo-electron microscopy structures of human OSTα/β in complex with cholesterols and an endogenous substrate, elucidating the structural basis for the function of OSTα/β. OSTα/β is assembled in a novel dimer-of-heterodimers manner: two OSTα units form the homodimeric core, with two OSTβ units bound to the periphery. OSTα adopts the G-protein-coupled-receptor (GPCR) fold, and contains a unique cysteine-rich loop with seven palmitoylation sites; these cooperate with transmembrane helices 5 and 6, constituting a BA recognition site. A positive cavity in OSTα connects the BA site, and facilitates the transmembrane translocation of BAs through OSTα/β. Together, this study reveals the architecture and transport mechanism of OSTα/β, and provides insights into the structure-function relationships of this crucial transporter in BA homeostasis.


