Spin-polarized oxygen evolution reaction under magnetic field
Date:28-05-2021 Print
The sluggish kinetics of oxygen evolution reaction (OER) is a major cause for the low of efficiency in techniques, such as solar water splitting, rechargeable metal-air batteries, regenerative fuel cells, and water electrolysis. Exploring better catalysts for OER has become increasingly attractive in the field of materials, chemistry, and energy in recent years. Non-precious 3d-transition metal oxides (TMOs), such as Fe-, Co-, and Ni-based oxides, are promising cost-effective catalysts. Their catalytical activities are tunable as the diversity in oxides families affords numerous freedoms to tailor their physicochemical properties. Typically,Eg occupancy in TMOs can be tuned the binding strength between the metal and the oxygen species, resulting in the OER activities. The optic and electronic fields are also applied to improve the OER activities by controlling the electron concentration and carries mobility. Most recently, the magnetic field as a new strategy has become increasingly attractive for promoting the enhancing OER. The spin states of electrons in the OER process and the kinetics mechanism in OER under a magnetic field becomes the key problems in the OER research field.
In recent years, Assoc. Prof. Haitao Yang from Group N04 in Institute of Physics of the Chinese Academy of Sciences and Prof. Zhichuan Xu from Nanyang Technological University have been cooperating together to study the spin polarization effect on the electro-catalysis. They investigated the local spin configurations of NixFe1-xOOH in experiment and theory and put forward the dependency between electron spin states and oxygen evolution and reduction reaction of the water cycling. They found that the spin-related electron transfer can switch the rate-determining step of OER on transition metal oxides (Adv. Mater. 32, 2003297(2020)).
Recently, they reported that the OER can be enhanced by using ferromagnetic ordered catalysts as the spin polarizer for spin selection under a constant magnetic field (Fig. 1). The HRTEM, Raman spectra, and XPS results indicated the magnetic catalyst is stable without no surface reconstruction (Fig. 2). The spin polarization occurs at the first electron transfer step in OER by the analysis of Tafel slope (Fig. 3), where coherent spin exchange happens between the ferromagnetic catalyst and the adsorbed oxygen species with fast kinetics, under the principle of spin angular momentum conservation. The calculation results demonstrated that in the next three electron transfer steps, as the adsorbed O species adopt fixed spin direction, the OER electrons need to follow the Hund rule and Pauling exclusion principle, thus to carry out spin polarization spontaneously and finally lead to the generation of triplet state O2 (Fig 4). More interesting, the current density of the ferromagnetic catalyst increases with the increase of the magnetic field strength and the OER performance of the ferromagnetic catalyst remains even after the magnetic field is removed (Fig. 5). This is because the magnetic moment is still aligned. The enhancing OER activity can’t be found in the non-magnetic catalysts at the same reaction condition. This finding showcase spin-polarized kinetics of oxygen evolution reaction, which gives references in the understanding and design of spin-dependent catalysts. This work will be helpful for further development of magnetic field assisted OER-enhancing strategy and related catalysts.
The results have been published on line in Nature Communications (Nat. Commun. 12, 2608 (2021)). Xiao Ren, Tianze Wu, Yuanmiao Sun contributed equally to this work. Haitao Yang and Zhichuan Xu are corresponding authors. The work at IOP is supported by grants from the National Natural Science Foundation of China (11274370 and 51471185), the National Key Research and Development Projects of China (2016YFJC20013, and 2018YFA0305800), and Authors in Singapore thank the support from the Singapore Ministry of Education Tier 2 Grants (MOE2017-T2-1-009 and MOE2018-T2-2–027).
Link of the article:
https://www.nature.com/articles/s41467-021-22865-y
https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202003297
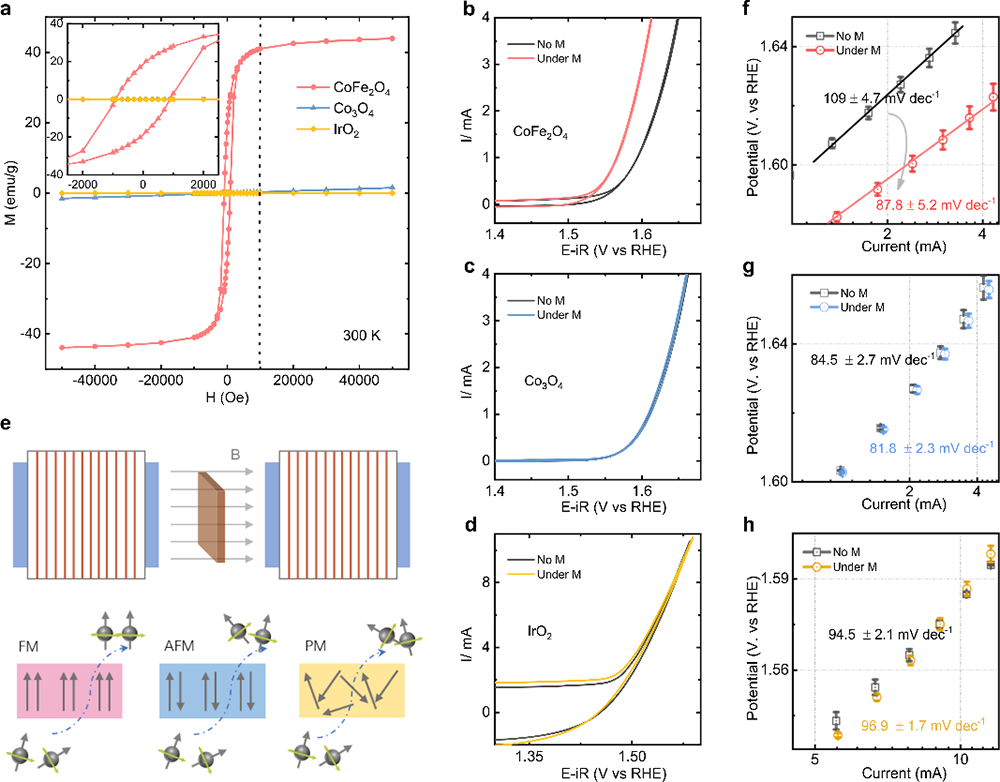
Fig. 1 Spin polarization promotes OER. a Magnetic hysteresis loops of CoFe2O4, Co3O4, and IrO2 powders at room temperature (300 K) and the magnified graph inset in the top left of this panel. (Here, IrO2 powder is a commercial catalyst (bulk, Premetek). Cyclic voltammetry (CV) of CoFe2O4. (b), Co3O4 (c), and IrO2 (d) catalysts at a scan rate of 10 mV/s in O2-saturated 1 M KOH with and without a constant magnetic field (10,000 Oe). e The schematic of the generation of the polarized electron under a constant magnetic field. The Tafel plots of CoFe2O4, (f) Co3O4 (g), and IrO2 (h) catalysts with and without a constant magnetic field (10,000 Oe). The error bar represents three independent tests.

Fig. 2 No surface restructuration on CoFe2O4. HADDF (a) and ABF (b) images of CoFe2O4 after OER. The line profiles of HADDF (c) acquired at the pink line rectangular zone. d Raman spectra of CoFe2O4 before and after OER. eThe Fe 2p, Co 2p, and O 1 s XPS spectra results of CoFe2O4 before and after OER.

Fig. 3 OER under the different temperature. a LSV curves of CoFe2O4 at a scan rate of 10 mV/s in O2-saturated 1 M KOH with and without a constant magnetic field (10,000 Oe) under the different temperatures (room temperature (RT): ~303 K, 308 K, 318 K, and 328 K). The corresponding Tafel plots are shown in b. c LSV curves of Co3O4 with and without a constant magnetic field (10,000 Oe) under the different temperatures (room temperature (RT): ~303 K, 313 K, and 323 K). The corresponding Tafel plots are shown in d. e LSV curves of IrO2 with and without a constant magnetic field (10,000 Oe) under the different temperatures (room temperature (RT): ~303 K, 313 K, and 323 K). The corresponding Tafel plots are shown in f. Tafel slopes at various temperatures are summarized in g.
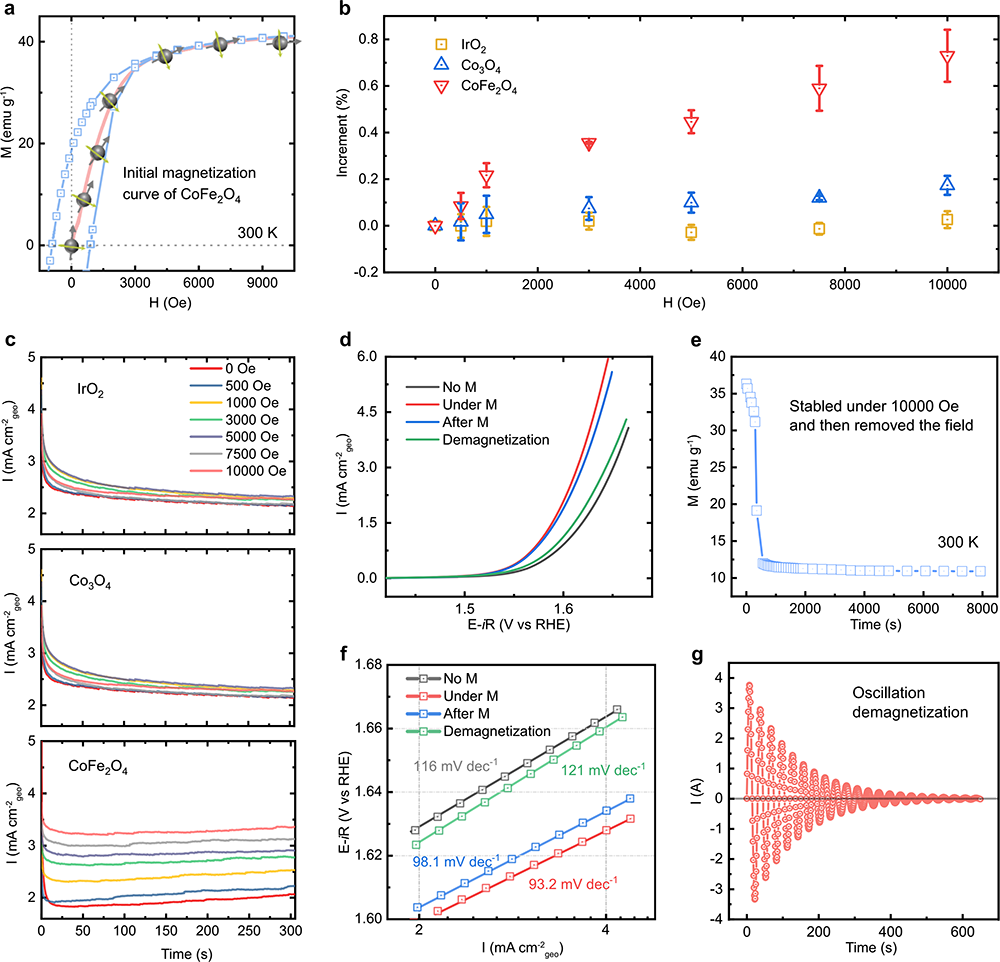
Fig. 4 The effect of gradient magnetic field, remanence, and demagnetization. a Initial magnetization curve of CoFe2O4. b CA test in 1 M KOH under the different magnetic field strength (0, 500, 1000, 3000, 5000, 7500, and 10000 Oe) at a constant potential of 1.66 V versus RHE for CoFe2O4, Co3O4, and 1.56 V versus RHE for IrO2. c The increment of the current density under different magnetic field strength. It was calculated by the following equation: Increment (%) = (jM – jM=0)/jM=0; jM is the chronopotentiometry current density values obtained under the applied magnetic fields (0, 500, 1000, 3000, 5000, 7500, and 10000 Oe). The error bar represents three independent tests. d LSV curves of CoFe2O4 at a scan rate of 10 mV/s in O2-saturated 1 M KOH with and without a constant magnetic field (10,000 Oe), after the magnetic field removed (after M), and after demagnetization. The corresponding Tafel plots are shown in f. e The magnetization of CoFe2O4 after removing a constant magnetic field of 10,000 Oe. g The curve of demagnetization for CoFe2O4.

Fig. 5 Spin-polarized OER. a The projected density of states (PDOS) of CoFe2O4 without and with spin alignment. b The spin density for CoFe2O4 with and without spin alignment. c Schematic of spin-exchange mechanism for OER. The first electron transfer step is promoted by spin polarization through the FM exchange (QSEI), which gives smaller electronic repulsions and makes the adsorbed O species have a fixed spin direction. d The calculated Pourbaix diagram of the (111) surface of CoFe2O4. e The spin-polarization mechanisms in OER with starting from the step of O* + OH- → *OOH + e- step. f The free energy diagram of OER at 1.23 V (vs. RHE)61,62 with and without the spin alignment on the (111) surface of CoFe2O4 toward triplet oxygen production.


