Chinese Scientists Discover a New Class of Viscoelastic Inorganic Glass (VIGLAS) Electrolytes for Solid-State Batteries
Date:01-10-2023 Print
Research Background
Solid-state batteries, often hailed as the next disruptive battery technology, offer a promising solution to the safety concerns associated with conventional liquid lithium-ion batteries while significantly enhancing energy density. This technological advancement holds the potential to revolutionize key industries, including electric vehicles, energy storage, and mobile devices. However, the practical implementation of solid-state batteries is hindered by several limitations related to interface stability and manufacturing costs. For instance, organic polymer solid-state batteries demonstrate impressive mechanical stability at the interface, but they fall short in terms of chemical stability. This limitation restricts their energy density, primarily due to compatibility issues with high-voltage cathodes. On the other hand, commercially viable inorganic sulfide solid-state batteries, while showing promise, come with the drawback of high manufacturing costs. Moreover, they require operation under extremely high pressures, often reaching several tens of atmospheres, presenting substantial challenges on the path to commercialization. Hence, the quest for a novel electrolyte material that can effectively address these issues becomes paramount in the pursuit of advancing solid-state battery technology.
Introduction to the Work
Recently, a significant breakthrough in the field of solid-state batteries has been achieved by the research team led by Prof. Yong-Sheng Hu at the Institute of Physics, Chinese Academy of Sciences. Their research on a novel viscoelastic inorganic glass (VIGLAS) electrolyte has been published in the top-tier energy journal "Nature Energy."
The team has successfully accomplished the transformation of delicate room-temperature molten salts, namely LiAlCl4 and NaAlCl4, into viscoelastic glasses, denoted as LiAlCl2.5O0.75 (LACO) and NaAlCl2.5O0.75 (NACO), by introducing oxygen atoms in place of certain chlorine atoms. What sets this material apart is its remarkable ability to bend and fold with ease at room temperature, challenging the previously held assumption that inorganic solid electrolytes couldn't exhibit the mechanical flexibility associated with organic electrolytes. This groundbreaking discovery paves the way for an entirely new frontier in the development of solid-state electrolytes.
The research team has unveiled both the formation and ion-conduction mechanisms of these inorganic glasses, known as VIGLAS materials. These materials exhibit a glass transition temperature (Tg) lower than room temperature, which results in a viscoelastic behavior reminiscent of polymers under ambient conditions. This low Tg can be attributed to the balanced oxygen-to-chlorine ratio, where oxygen bridges play a crucial role in forming appropriately sized Al-O-Al networks. These networks, in turn, limit atomic rearrangement during condensation. Furthermore, the presence of trace amounts of uncoordinated LixAlCl3+x, acting as "plasticizers," contributes to the reduction of Tg. Regarding ion conductivity, the oxygen bridges in VIGLAS materials shorten the distance between Li-Li pairs, thereby facilitating Li+ ion hopping. Additionally, VIGLAS electrolytes exhibit chain segment motion similar to that observed in PEO polymer electrolytes. This motion promotes collective Li+ ion migration in the vicinity, enhancing ion conductivity.
Importantly, the VIGLAS not only exhibit remarkable deformability comparable to organic polymers but also inherit the desirable traits of conventional inorganic electrolytes. These traits include a high voltage tolerance of up to 4.3 V and impressive ionic conductivity exceeding 1 mS/cm. This inherent advantage effectively resolves the challenges related to both mechanical and chemical stability at the positive electrode interface in solid-state batteries. Consequently, it enables the groundbreaking achievement of true ambient-temperature operation in inorganic all-solid-state lithium and sodium batteries without the need for external pressure (maintained at < 0.1 MPa).
Currently, the commercialization of solid-state batteries faces significant challenges associated with high manufacturing costs and complex production processes. Fortunately, this research presents an ideal solution. Firstly, the production cost of this innovative solid-state electrolyte material is exceptionally low, primarily because its core component is the abundant aluminum element found in the Earth's crust. This results in material costs of only $6.85 per kilogram for LACO and a mere $1.95 per kilogram for NACO. These costs represent a mere fraction, at 2% and 0.6%, respectively, of the current mainstream Li6PS5Cl solid-state electrolyte, which comes with a high price of $319 per kilogram. Another merit is these VIGLAS boast a low melting point, below 160℃, allowing them to effectively infiltrate porous electrodes like a liquid when subjected to suitable heating conditions. This capability enables the achievement of commercial cathode loadings that exceed 20 mg/cm2. Furthermore, these materials exhibit ductility similar to organic polymers, enabling the manufacture of large-area electrolyte films using techniques such as roll-to-roll processes. These remarkable characteristics render this novel solid-state electrolyte material highly competitive in terms of both material and manufacturing costs, making it an ideal choice for addressing the challenge of pressure-free operation in all-solid-state battery cathodes.
This work was supported by the National Natural Science Foundation (NSFC) of China (52122214) and Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020006).
Paper information:
Tao Dai, Siyuan Wu, Yaxiang Lu*, Yang Yang, Yuan Liu, Chao Chang, Xiaohui Rong, Ruijuan Xiao, Junmei Zhao*, Yanhui Liu, Weihua Wang, Liquan Chen & Yong-Sheng Hu*. Inorganic glass electrolytes with polymer-like viscoelasticity. Nature Energy (2023, https://doi.org/10.1038/s41560-023-01356-y).
Paper link:
https://doi.org/10.1038/s41560-023-01356-y
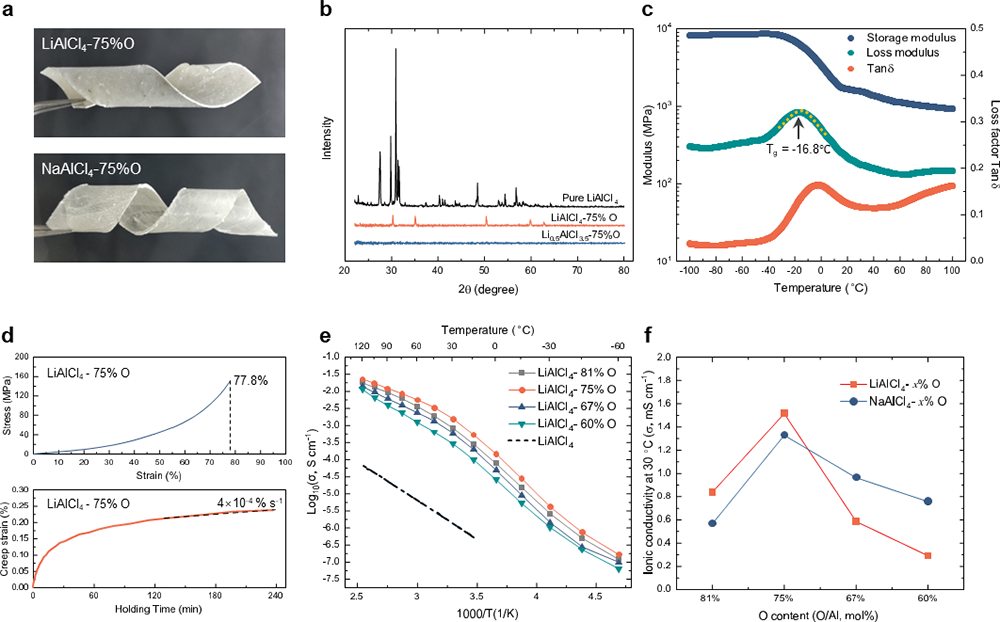
Figure 1 The mechanical properties and ionic conductivities of LACO and NACO.
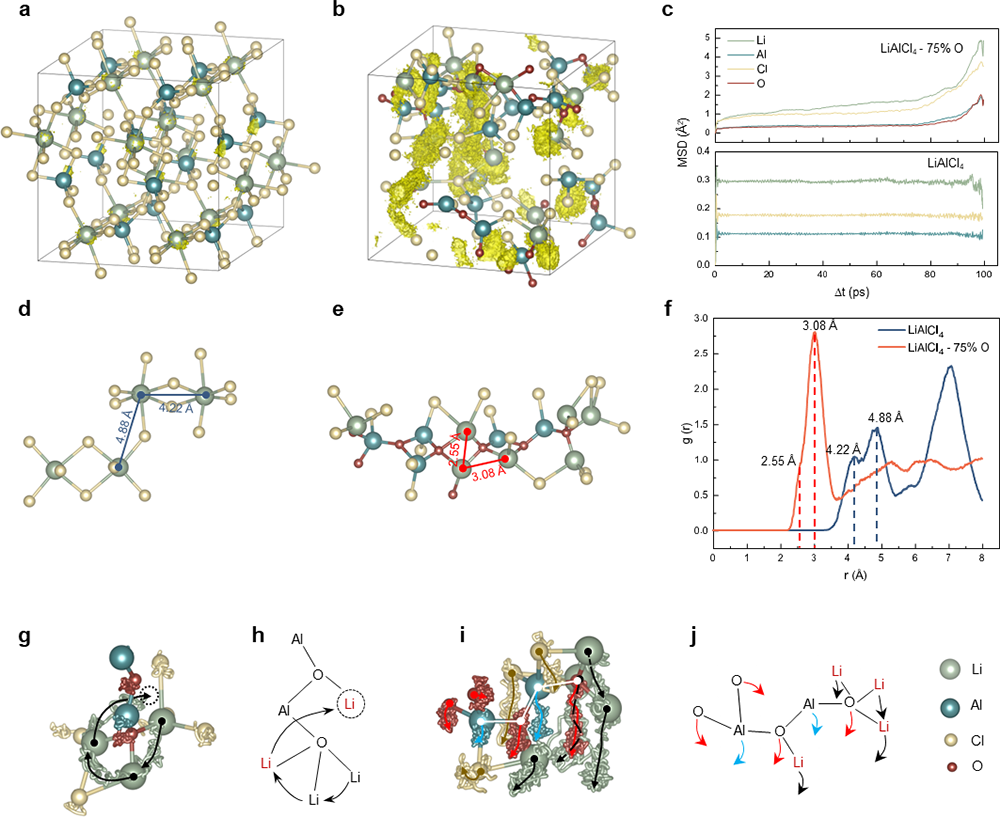
Figure 2 Two ion transport mechanisms in LACO.
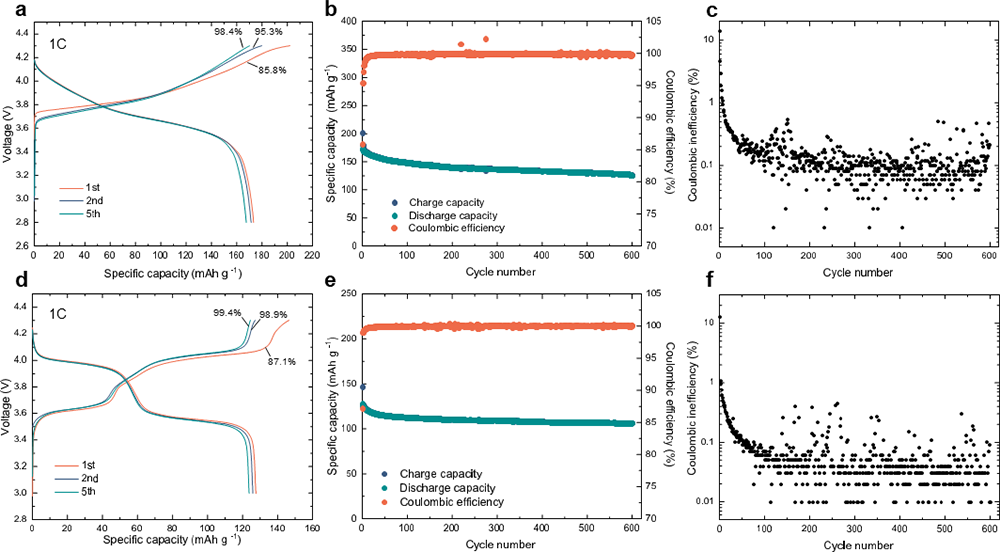
Figure 3 Cycling performance of Li- and Na-based all-solid-state batteries without additional stacking pressure.
Contact:
Institute of Physics
Yong-Sheng Hu
Email:yshu@iphy.ac.cn
Key words:
Viscoelastic inorganic glass; solid state electrolyte; solid state battery
Abstract:
Solid-state batteries offer an alternative promising power source for electric vehicles. However, the interfacial mechanical stability of inorganic electrolytes is inferior to that of organic electrolytes. A high stack pressure (several to hundreds of megapascals) is often required to maintain intimate contact with electrodes. Here we report a class of viscoelastic inorganic glass (VIGLAS) to serve as solid electrolytes by simply replacing chlorine of tetrachloroaluminates with oxygen. The VIGLAS possesses high ionic conductivity (~1 mS cm-1 at 30°C) for both Li+ and Na+ , superior chemo-mechanical compatibility with 4.3 V cathodes as well as the ability to enable pressure-less Li- and Na-based solid-state batteries (<0.1 MPa). The low melting temperature (<160°C) allows the electrolytes to efficiently infiltrate electrode materials, akin to a liquid battery. Additionally, the deformability of the electrolytes facilitates the feasibility of scale-up through the production of thin films via a rolling process.


